| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- 少见的肾脏肿块(新加免疫组化公布)
| 姓 名: | ××× | 性别: | 男 | 年龄: | 65 |
| 标本名称: | 左肾脏 | ||||
| 简要病史: | 肉眼血尿两天,影像学提示左肾脏占位。手术切除肾脏,发现腹主动脉旁有核桃大肿块,临床怀疑为肿大淋巴结。 | ||||
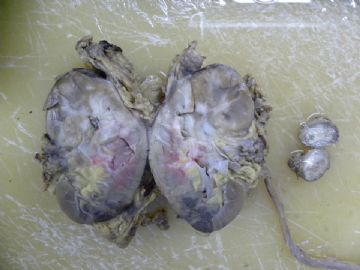
| 肉眼检查: | 左肾脏肿大,切面以肾盂为中心有4X4cm不规则灰白区,质硬,与正常肾组织无明显界限。腹主动脉旁肿块切面同肾脏。取材:1.肾被膜处;2.病变与正常组织交界处;3.灰白病变处;4.腹主动脉旁肿块。 | ||||
应金老师的提议,我们加做了HMB45,CD10,遗憾的是我科室没有Calponin。结果如图,请各位老师点评。
-
本帖最后由 于 2010-06-05 16:27:00 编辑

- 许春雷
-
liguoxia71 离线
- 帖子:4174
- 粉蓝豆:3122
- 经验:4677
- 注册时间:2007-04-01
- 加关注 | 发消息
-
本帖最后由 于 2010-06-06 12:04:00 编辑
CKpan、CK7、vimentin、S100淋巴结内大部分梭形细胞阳性,EMA淋巴结内部分梭形细胞阳性(+/-),
有意思的是p63似为胞浆定位,不知道是特异性还是非特异性。
Antibodies targeting p63 react specifically in the cytoplasm of breast epithelial cells exhibiting secretory differentiation
Antibodies targeting p63 react specifically in the cytoplasm of breast epithelial cells exhibiting secretory differentiation
Methods: Thirty breast specimens were tested immunohistochemically for p63 protein. These included seven with benign secretory changes, 10 secretory carcinomas (nine invasive), one microglandular adenosis, three lobular neoplasias, four invasive ductal carcinomas, three clear cell carcinomas, one squamous cell carcinoma and one mucinous carcinoma.
Results: Only cells exhibiting secretory changes or secretory carcinoma were cytoplasmically reactive for p63. The positive reaction was also present as an intraluminal secretory product. This reaction was not seen in cells undergoing apocrine differentiation or in other cells containing secretory vacuoles.
Conclusions: Cells with secretory changes contain p63 protein or an antigenic equivalent. The detection of p63 protein continues to have considerable value for the identification of myoepithelial cells and thus the determination of invasion, but will also have value in the determination of secretory carcinomas of the breast and in understanding their development.
Date of submission 27 January 2005 Accepted for publication 5 April 2005
Aberrant Cytoplasmic Expression of p63 and Prostate Cancer Mortality
Protein expression of p63 is used to differentiate prostate cancer from benign mimickers. Recent studies suggest that it may also distinguish aggressive prostate cancer with down-regulated expression occurring in men with more advanced disease. We conducted a prospective study among 298 men ages 51 to 84 years who were diagnosed with prostate cancer in the Physicians' Health Study in 1983 to 2004 and whose tissue was available for immunohistochemical staining. We used Cox proportional hazards regression to evaluate the association of p63 protein expression with fatal prostate cancer. We correlated p63 expression with tumor cell proliferation (Ki-67) and apoptosis (TUNEL staining). The predominant location of tumor p63 staining occurred in the cytoplasm, an uncommon departure from the strong nuclear staining usually observed in nonneoplastic basal cells. Increasing expression of cytoplasmic p63 (tertiles) was associated with prostate cancer mortality (n = 19 deaths); the hazard ratios (95% confidence intervals) were 1.0 (reference), 4.0 (0.9-18.9), and 5.9 (1.3-27.5; Ptrend = 0.03). The positive trend remained significant (P = 0.047) after multivariable adjustment for age, year of diagnosis, and Gleason score. Higher tertiles of cytoplasmic p63 were also associated with reduced levels of apoptosis (Ptrend = 0.0408) and increased cellular proliferation (Ptrend = 0.0026). We found aberrant expression of p63 in the cytoplasm to be associated with increased prostate cancer-specific mortality up to 20 years after diagnosis. The mislocalized expression was associated with reduced apoptosis and higher proliferative activity and may suggest an oncogenic role in prostate cancer progression and survival.
(Cancer Epidemiol Biomarkers Prev 2009;18(2):595–600)
-
本帖最后由 于 2010-06-02 22:55:00 编辑
S-100和Vimentin应该判断为阳性。
肾脏肿瘤往往既表达CK又表达Vimentin; 而S-100一般认为是周围神经、黑色素细胞、肌上皮细胞、脂肪细胞、颗粒细胞等的标记物,但平滑肌、嗜铬细胞瘤中支持细胞、有些神经内分泌细胞也可表达S-100。因此,S-100的特异性差,确定其意义必须以形态为基础。
建议再标记HMB-45、CD10、Calponin 是为进一步排除恶性黑色素瘤/软组织透明细胞肉瘤、肌上皮瘤等。
根据此例形态特点、IHC CK与Vimentin 双表达,尤其是肾小球完好,而周围肾小管区均为肿瘤,高度提示为集合管癌,并且又有p63+支持, 只要能除外转移性恶性肌上皮瘤,诊断就能基本确定。临床恶性度高,已有淋巴结转移,也比较符合集合管癌的生物学行为。
仅供参考,谢谢!

- xljin8
-
本帖最后由 于 2010-06-02 20:36:00 编辑
1)p63+ -- 集合管癌?
2)肌上皮癌?转移性或原发性?
3) IHC再标记:HMB-45、Calponin、CD10 WT-1
4)参考 Am J Surg Pathol 最新文献,少数集合管癌 p63可阳性(14%)
Albadine R, Schultz L, Illei P, Ertoy D, Hicks J, Sharma R, Epstein JI, Netto GJ.
PAX8 (+)/p63 (-) Immunostaining Pattern in Renal Collecting Duct Carcinoma (CDC):A Useful Immunoprofile in the Differential Diagnosis of CDC Versus UrothelialCarcinoma of Upper Urinary Tract. Am J Surg Pathol. 2010 May 11. [Epub ahead of print]Departments of *Pathology double daggerUrology section signOncology, JohnsHopkins University, Baltimore, MD daggerDepartment of Oncology, HacettepeUniversity, Ankara, Turkey.
rare but aggressive type of renal malignancy with variable morphologic features.
One of the World Health Organization diagnostic criteria for CDC is the exclusion of
urothelial carcinoma of renal pelvis from the differential diagnosis. PAX8 is a novellineage restricted transcription factor expressed in renal tubules. We investigated the
expression pattern of PAX8 in CDC and its utility, in combination with p63, in resolving
the differential diagnosis of CDC versus upper tract urothelial carcinoma (UUC).
DESIGN: Archival tissues from 21 CDC and 34 UUC were retrieved from our institutional
files. Immunohistochemistry for PAX8 and p63 were performed on routine and tissue
microarray sections using standard immunohistochemistry protocol. Intensity of nuclear
staining was evaluated for each marker and assigned an incremental 0, 1+, 2+, and 3+ score. Extent
of staining was categorized as focal (<25%), nonfocal (25% to 75%), or diffuse (>75%).
RESULTS: CDC: All 21 (100%) CDC were positive for PAX8. Intensity of expression
was moderate to strong (2+/3+) in 19 cases (90%). Extent of staining was diffuse
in 13 of 21 tumors. The p63 was positive in 3 of 21 (14%) CDC cases (PAX8+/p63+).
UUC: The 34 UUC included 5 pT1, 4 pT2, and 25 pT3/pT4 tumors.Thirty-one of 34 (91.2%)
UUC were negative for PAX8, whereas 33 of 34 (97%) were p63 positive. Staining
intensity was moderate in 15 cases (44%), of which 12 were nonfocal or diffuse.
The unique p63-negative UUC was a pT1 tumor that was also negative for PAX8 (PAX8-/p63-).
CONCLUSIONS: We propose the use of the combination of PAX8 and p63 in the diagnosis of poorly
differentiated renal sinus epithelial neoplasms where the differential diagnosis includes
CDC versus UUC.The immunoprofile of PAX8+/p63- supports the diagnosis of CDC with a sensitivity
of 85.7% and a specificity of 100%. In contrast, a (PAX8-/p63+) profile supports the diagnosis
of UUC with a sensitivity of 88.2% and a specificity of 100%. The inverse PAX8/p63 expression
seen in CDC and UUC supports a renal tubular rather than an urothelial differentiation in CDC
given the nephric lineage restriction f PAX8.
- xljin8



 谢谢楼主
谢谢楼主