| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
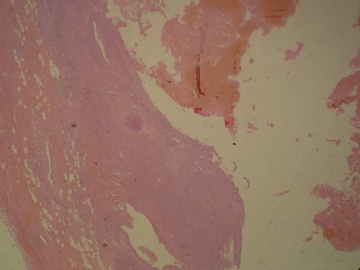
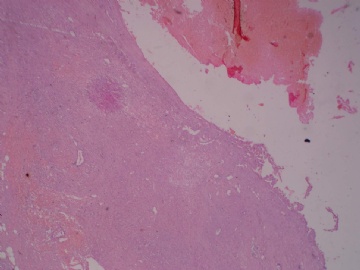
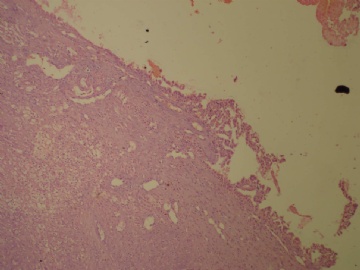
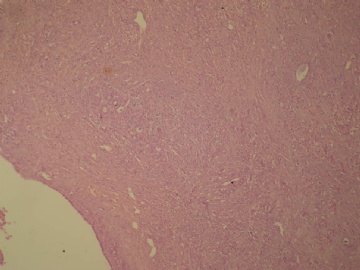
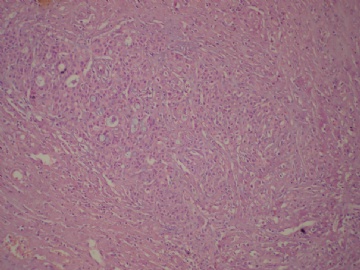
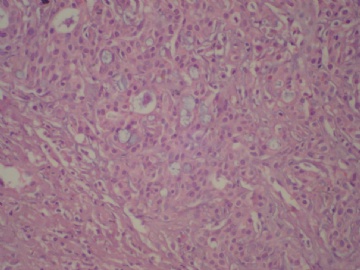
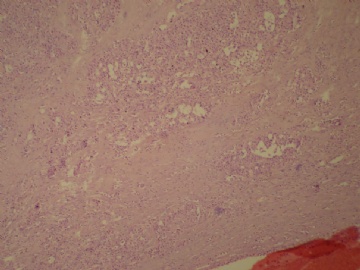
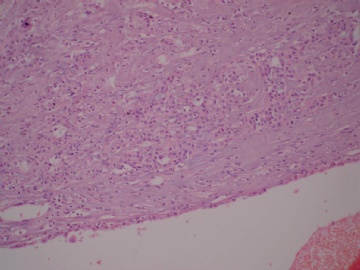
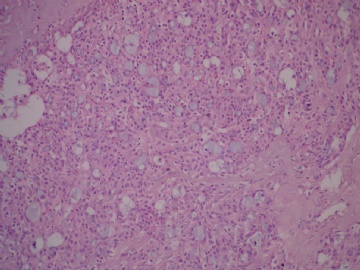
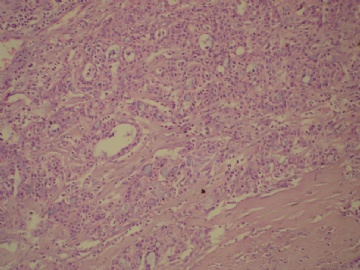
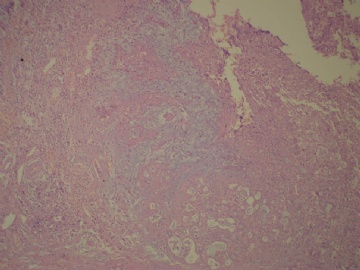
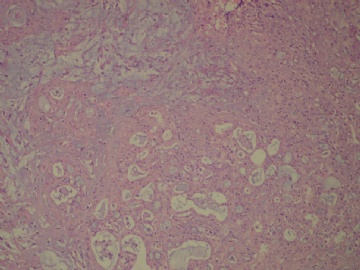
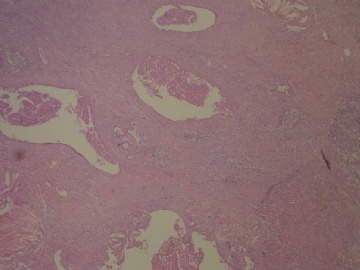
- B3160右乳包块(镜下富于粘液)
| 姓 名: | ××× | 性别: | 女 | 年龄: | 72 |
| 标本名称: | 右乳包块 | ||||
| 简要病史: | 发现右乳包块1月余 | ||||
| 肉眼检查: | 约5X3.8X2cm大,囊实性 | ||||
相关帖子
- • 乳头血性溢液,乳管内肿物,请看免疫
- • 乳腺肿瘤?
- • 左乳肿块
- • 边界清楚的乳腺包块
- • 乳腺肿块,请会诊
- • 乳腺癌?
- • 左乳腺肿块,新加免疫组化
- • 左乳肿块,新加免疫组化
- • 乳腺包块-请会诊
- • 左乳肿块
| 以下是引用alliwantisyou在2011-3-30 17:45:00的发言:
上级医院会诊:囊内乳头状癌伴局部浸润 IHC:SMA、S-100、Calponin均(—),其它未标记 我们科室考虑的是囊内乳头状癌,分泌性癌,腺泡细胞癌,但最终以囊内乳头状癌(或称包裹性乳头状癌)伴来源于囊内乳头状癌的浸润性癌(直径<1cm)发出报告 刘彤华的《诊断病理学》里面讲到囊内乳头状癌可以有细胞外粘液及细胞内粘液(粘液化癌),神经内分泌分化,但文献里面好像把这些特征归在了实性乳头状癌里面,刘彤华那本书很简略未细讲。但本例似乎和我搜索到的所有实性乳头状癌均不一样! 本例形态学真的很像分泌性癌或腺泡细胞癌(有文献报道它们有可能是同一种实体,至少很相似,是重叠的),前者可以有很多原位分泌性癌成分。分泌性癌或腺泡细胞癌结构均能以乳头状、甲状腺滤泡样、筛状、微囊、细胞外细胞内分泌物等形态多少不等分布于肿瘤实体内。本例大体和镜下确有囊+乳头结构。 S-100在分泌性癌的表达文献报道也不一致,有些报道肿瘤腺上皮细胞弥漫强阳性表达,有的报道为阴性 总之:本例不管最终是上面所述的何种肿瘤,它们均是低度恶性的肿瘤,即使有浸润成分存在也是如此。最终谁知道是什么呢?以上仅供交流学习 |
Thank for sharing.
My main differential diagnoses are 包裹性乳头状癌 伴浸润性癌 and 分泌性癌.
Myoepithelial stains can be important for dx.
这个例子刚刚读片会参加完。P63、SMA、Calponin、CK5/6均阴性,S-100(++)、CK(高分子量)(++),最终诊断符合分泌性癌
分泌性癌重要文献摘引:
Secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to the basal-like carcinoma spectrum
Presented in part at the Annual United States and Canadian Academy of Pathology, Denver, 2008.
Marick Laé1, Paul Fréneaux1, Xavier Sastre-Garau1, Olfa Chouchane1, Brigitte Sigal-Zafrani1 and Anne Vincent-Salomon1
Abstract
Secretory breast carcinomas (<0.15% of breast tumors) are associated with a characteristic morphology and a favorable prognosis. Remarkably, this entity is the only epithelial tumor of the breast with a balanced translocation, t(12;15), that creates an ETV6-NTRK3 gene fusion encoding chimeric tyrosine kinase also encountered in cellular mesoblastic nephroma and infantile fibrosarcoma. The aim of this study was to determine the phenotypic class (ie luminal A/B, ERBB2, basal-like) of secretory breast carcinoma. A series of six secretory breast carcinomas were identified in our files. The ETV6 rearrangement was confirmed in all cases by fluorescence in situ hybridization. Immunophenotype was assessed with anti-ER, PR, ERBB2, KIT, EGFR, E-cadherin, vimentin, PS100, smooth muscle actin, basal (CK5/6 and 14), luminal cytokeratins (CK8/18) and p63 antibodies. In situ and invasive components shared the same immunoprofile and were ER, PR, ERBB2 negative with expression of basal cytokeratins. ETV6 gene alterations were present in both in situ and invasive components, highlighting their genetic similarities. The immunoprofile data (triple-negative with expression of basal markers) showed that secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to the phenotypic basal-like spectrum of breast carcinomas. These results support the hypothesis that secretory breast carcinomas have immunohistochemical and genetic features that distinguish them from other basal-like tumors of the breast.
Secretory breast carcinoma is one of the rarest types of breast cancer, accounting for less than 0.15% of all breast cancers.1 This entity was initially termed ‘Juvenile breast carcinoma’ by McDivitt and Stewart,2 as the average age of the seven patients in their series was 9 years, with a range of 3–15 years. More cases in children and adults have subsequently been described.3, 4 The age at presentation ranges from 3 to 87 years with a median of 25 years.1 It was therefore recommended to replace the term ‘juvenile breast carcinoma’ by the descriptive term ‘secretory breast carcinoma’.
Secretory breast carcinomas are distinguished from invasive ductal carcinomas of the breast by their characteristic histomorphology. Secretory breast carcinomas can demonstrate several histologic patterns including, solid, microcystic and tubular, and many tumors contain all three patterns. Tumor cells are polygonal with granular eosinophilic cytoplasm. Atypia is minimal or absent and mitotic activity is low. A typical finding is the presence of intracellular and extracellular secretory material (periodic acid-Schiff and Alcian blue positive).
Tognon et al5 recently showed that secretory breast carcinomas are associated with a characteristic balanced translocation, t(12;15), that creates a ETV6-NTRK3 gene fusion. The fusion between ETV6 (TEL) transcription factor and the NTRK3—neurotrophin-3 tyrosine kinase protein occurs in various cell types and lineages.5 This specific translocation was first cloned in pediatric mesenchymal cancers: congenital fibrosarcoma and congenital cellular mesoblastic nephroma, two morphologically similar pediatric mesenchymal tumors with no epithelial features.6, 7 The biological consequence of this translocation is the expression of a chimeric tyrosine kinase protein with a potent transforming activity in very different cell lineages including fibroblasts, and breast epithelial cells.8 The finding of a fusion transcript in secretory breast carcinomas and the demonstration that ETV6-NTRK3 can transform normal murine mammary epithelial cells strongly suggest that this chimeric protein constitutes a primary genetic lesion in this subtype of breast carcinoma.8 The ETV6-NTRK3 fusion activates RAS-MAP kinases and PI3K–Akt pathways which are important for breast cell proliferation and survival.8
Gene expression profile analysis has recently identified at least six different breast carcinoma types: luminal A, B, C, ERBB2, basal-like and normal-like types.9, 10 Subsequent immunophenotype analyses provided surrogate markers to identify these molecular types in clinical practice.11, 12
Secretory breast carcinoma is a paradoxical entity harboring a translocation known to be oncogenic in two other tumor types, including a mesenchymal tumors, while also demonstrating true epithelial differentiation with secretory activity. In this context, the aim of this study was to identify the secretory breast carcinoma phenotypic class. Six histologically and molecularly confirmed secretory breast carcinomas were evaluated. Secretory breast carcinomas were found to be low-grade triple-negative (ER, PR and ERBB2 negative) carcinomas that express basal-cell markers. This observation suggests that secretory breast carcinomas with ETV6-NTRK3 fusion gene are part of the basal-like spectrum. ETV6 gene alterations were present in both the in situ and invasive components, highlighting their genetic similarities. These results also show that secretory breast carcinomas have genetic features that distinguish them from other basal-like tumors of the breast.
Pathologic Findings
Tumor size ranged from 5 to 40 mm, with a mean of 17 mm. Microscopically, all tumors presented both invasive and in situ components. Tumors demonstrated several histologic patterns including, solid, microcystic, and tubular, and many tumors contained all three patterns. Tumor cells were polygonal with granular eosinophilic cytoplasm. Atypia was moderate to marked. Nuclei were ovoid with small nucleoli. Mitotic activity was zero or low (mitotic activity ranged from 0 to 8/10 HPF). A typical finding was the presence of intracellular (intracytoplasmic lumina) and extracellular secretory material (PAS and Alcian blue positive). Four tumors were Elston and Ellis grade 2 (66%) and two were grade 1 (33%). Vascular invasion was present in one case. Perineural infiltration was absent in all cases. Necrosis was absent in the invasive component. All tumors presented an in situ secretory component with cribriform, microcystic or massive architecture at the margins or within the tumor. In situ nuclear grade was intermediate without necrosis except for one tumor which presented a high-grade in situ component with necrosis. Pathologic findings are reported in Table 2 and illustrated in Figure 1a–c.
Immunohistochemical Findings
Immunohistochemical findings are reported in Table 3 and illustrated in Figure 1d–i. All tumors were negative for ER, PR and ERBB2. All tumors expressed CK8/18 at least focally (1–80% of cells). E-cadherin expression (membranous immunoreactivity of 5–90% of cells) was present in all tumors. Five tumors (83%) expressed CK5/6 (1–80% of cells), vimentin (5–60% of cells). Four tumors focally expressed KIT (1–5% of cells). CK14 expression was present in two tumors and EGFR expression was present in three tumors. Five tumors (83%) expressed S-100 (strong and diffuse expression in 100% of cells) with focal smooth muscle actin expression (2–10% of cells). None of the tumors cells expressed p63. In situ and invasive components presented the same immunoprofile.