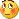| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- 左额叶脑肿块(诊断?)请各位老师发表高见。
| 姓 名: | ××× | 性别: | 女 | 年龄: | 70岁 |
| 标本名称: | 左额叶病变组织 | ||||
| 简要病史: | 头部不适伴语言迟缓7天入院。 | ||||
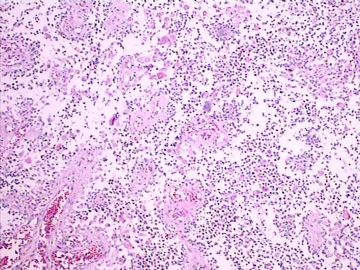
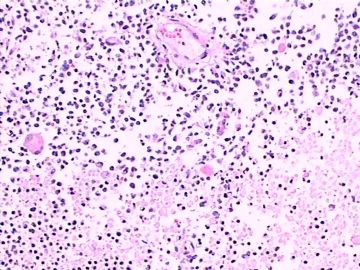
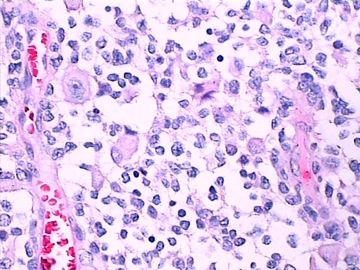
| 肉眼检查: | 肿瘤组织3块,大小共4.2x2.2x0.6cm,切面灰红,质软。 | ||||
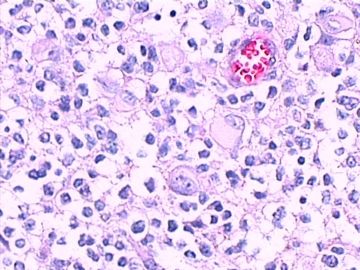
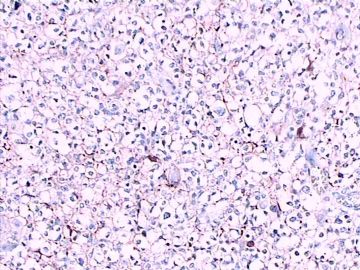
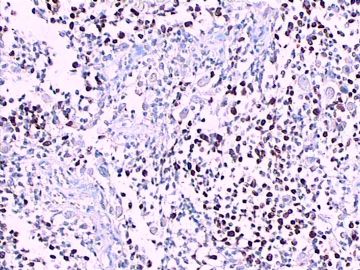
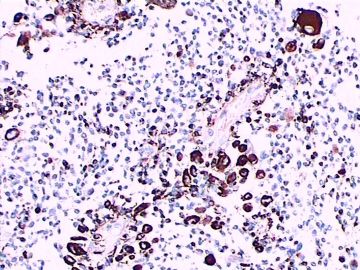
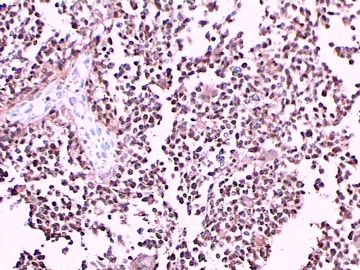
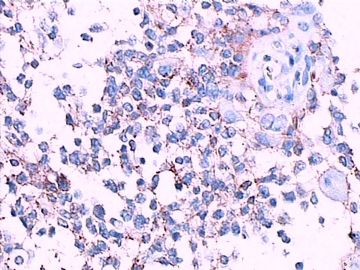
免疫组化染色:GFAP胞浆丰富的大细胞阳性,Olig2小细胞阳性,Syn阳性,S-100阳性,MBP部分细胞阳性,Neun局部散在少量大细胞阳性,需要除外残余神经元;Ki-67阳性率约40%。
11图MBP,12图Olig2,13图GFAP,14图S-100,15图Syn。
-
本帖最后由 于 2011-01-02 13:23:00 编辑
-
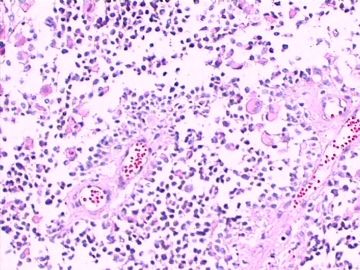
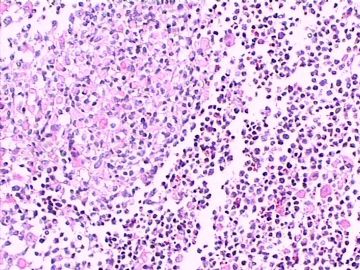
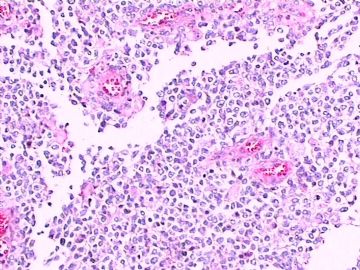
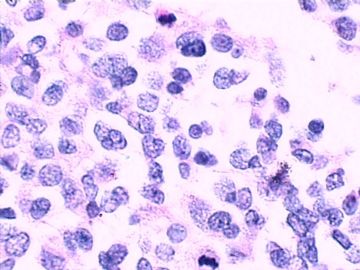
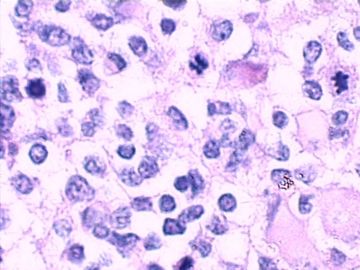
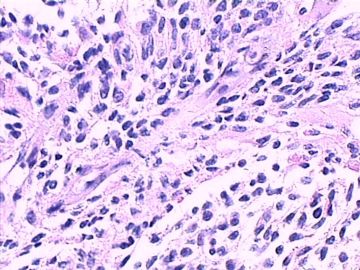
这不是少枝(突)胶质瘤,但细胞形态和组织结构有少枝胶质瘤样的表现。主要形态:(1)小血管增生比较明显,可见血管周假菊形团或假乳头状结构。(2)绝大多数瘤细胞为形态较一致的体积较小的细胞,易与少枝胶质瘤混淆。(3)这些小圆细胞胞核呈圆形或卵圆形,核染色质细密分布,少数瘤细胞可见核仁。这些细胞中意见核分裂。(4)一些视野下,可见神经节细胞样细胞,胞体大,胞浆丰富并嗜酸性着色,核院或卵圆形,核仁明显。(5)少数视野下(图10)显示瘤细胞呈长梭形。(6)IHC标记突出显示节细胞样细胞和小细胞均为Syn和S-100阳性,GFAP主要为大细胞阳性,Olig2主要为小细胞阳性,MBP有阳性,Neun局灶大细胞阳性。没有染CgA和CK。
综上所述,考虑为中枢神经细胞瘤,伴有节细胞,WHOII级。由于含有节细胞,是否也可以称为中枢节细胞神经细胞瘤。
结合临床与病理资料,需要与少枝胶质瘤、室管膜瘤和松果体细胞瘤等类型肿瘤鉴别。

- 王军臣
-
本帖最后由 于 2011-01-03 19:54:00 编辑
1)本病例有坏死、明显的核分裂象、Ki-67 指数40%,因此定位于WHO3级的脑肿瘤比较合理。
2)组织学分类:根据形态和IHC标记基本特点,主要鉴别为少突胶质细胞伴神经细胞分化和节细胞胶质瘤;
3)少突胶质瘤中出现神经细胞有二种情况,1)肿瘤中残留的正常胶质细胞;2)肿瘤伴有神经细胞分化(oligodendroglioma with neurocytic differentiation)。
4)鉴别要点是肿瘤中神经细胞的数量、分布和形态。
5)本病例有坏死、核分裂多;Olig2+、Syn+、GFAP+、 Ki-67指数高,比较符合“间变性少突胶质细胞伴神经细胞分化, WHO3级)。
仅供参考。
谢谢!

- xljin8
| 以下是引用海上明月在2011-1-1 23:14:00的发言: 这不是少枝(突)胶质瘤,但细胞形态和组织结构有少枝胶质瘤样的表现。主要形态:(1)小血管增生比较明显,可见血管周假菊形团或假乳头状结构。(2)绝大多数瘤细胞为形态较一致的体积较小的细胞,易与少枝胶质瘤混淆。(3)这些小圆细胞胞核呈圆形或卵圆形,核染色质细密分布,少数瘤细胞可见核仁。这些细胞中意见核分裂。(4)一些视野下,可见神经节细胞样细胞,胞体大,胞浆丰富并嗜酸性着色,核院或卵圆形,核仁明显。(5)少数视野下(图10)显示瘤细胞呈长梭形。(6)IHC标记突出显示节细胞样细胞和小细胞均为Syn和S-100阳性,GFAP主要为大细胞阳性,Olig2主要为小细胞阳性,MBP有阳性,Neun局灶大细胞阳性。没有染CgA和CK。 对:一些视野下,可见神经节细胞样细胞,胞体大,胞浆丰富并嗜酸性着色,核院或卵圆形,核仁明显。但这些大细胞GFAP阳性;NeuN阴性呀。大细胞和小细胞均为Syn和S-100阳性。是否是间变型星形细胞瘤? |
针对该病例,发表一下个人意见供大家讨论,谢谢。
1.老年病例,亚急性病程。
2.无影像学资料,尚不能评述肿瘤精确部位和病变特征。
3.形态学改变:由小圆形、多形性瘤细胞构成,具有胶质瘤背景的恶性肿瘤。瘤细胞密集分布或绕血管周围排列形成血管性假菊形团样结构。瘤细胞由圆形至梭形或多形性,核分裂相明显,偶见核偏位、胞浆丰富、嗜酸性的瘤细胞散在分布或上皮样瘤细胞呈巢状分布。瘤组织内见血管内皮增生和片状坏死,偶见残存神经元散在分布。
形态学诊断首先考虑胶质母细胞瘤(WHO IV级),亚型分类:大多数人将它称为上皮样胶母或横纹肌样胶母。该病例我更愿意诊断上皮样胶母。
需鉴别的诊断:
1.最近文献报道的恶性胶质神经元肿瘤(或称间变性胶质神经元肿瘤或称恶性胶质瘤伴有PNET成分):该肿瘤的基本形态是有PNET的成分和胶母成分混合存在。该病例的细胞形态没有PNET样的改变。
2.伴有神经元分化的间变性少突胶质细胞瘤:非常罕见!该病例细胞形态表现为多形性,没有神经毡岛样结构和神经元性菊形团结构,因此可能性不大,最好做一下1p/19q LOH检测和p53蛋白免疫组化。
3.至于大家提出的节细胞神经细胞瘤、中枢神经细胞瘤等,根据病人的年龄、部位和病理形态均不符合。
-
本帖最后由 于 2011-01-12 13:03:00 编辑
| 以下是引用yinwang在2011-1-11 11:01:00的发言:
针对该病例,发表一下个人意见供大家讨论,谢谢。 1.老年病例,亚急性病程。 2.无影像学资料,尚不能评述肿瘤精确部位和病变特征。 3.形态学改变:由小圆形、多形性瘤细胞构成,具有胶质瘤背景的恶性肿瘤。瘤细胞密集分布或绕血管周围排列形成血管性假菊形团样结构。瘤细胞由圆形至梭形或多形性,核分裂相明显,偶见核偏位、胞浆丰富、嗜酸性的瘤细胞散在分布或上皮样瘤细胞呈巢状分布。瘤组织内见血管内皮增生和片状坏死,偶见残存神经元散在分布。 形态学诊断首先考虑胶质母细胞瘤(WHO IV级),亚型分类:大多数人将它称为上皮样胶母或横纹肌样胶母。该病例我更愿意诊断上皮样胶母。 需鉴别的诊断: 1.最近文献报道的恶性胶质神经元肿瘤(或称间变性胶质神经元肿瘤或称恶性胶质瘤伴有PNET成分):该肿瘤的基本形态是有PNET的成分和胶母成分混合存在。该病例的细胞形态没有PNET样的改变。 2.伴有神经元分化的间变性少突胶质细胞瘤:非常罕见!该病例细胞形态表现为多形性,没有神经毡岛样结构和神经元性菊形团结构,因此可能性不大,最好做一下1p/19q LOH检测和p53蛋白免疫组化。 3.至于大家提出的节细胞神经细胞瘤、中枢神经细胞瘤等,根据病人的年龄、部位和病理形态均不符合。 |

- 王军臣
| 以下是引用yinwang在2011-1-12 12:39:00的发言: 你提的问题非常好!我想这也是神经病理的难点之处。 SYN阳性,关键你要看它在什么部位,肿瘤细胞胞浆或突起内?还是在残存的神经元周围或神经毡样基质内?该病例肿瘤范围广,累及大脑皮层,因此SYN阳性并不奇怪,这也是胶质瘤弥漫性浸润,无边界的特点。如有可能请追加做一下p53蛋白。鄙人姓汪,名寅。不是王寅。期待p53 蛋白结果。谢谢! 嘿嘿,是我不好,打字过快,未经审阅就发帖子了,重大失误。特向汪寅教授表示深深歉意! 汪寅教授是我国著名的年轻有为临床神经病理学家。谢谢汪教授为我们解惑和指导! |

- 王军臣
| 以下是引用七公主在2011-1-12 18:12:00的发言: 不好意思,将汪老师名字打错了,已改正。不知P53蛋白在胶质母中的意义? 请见以下文献: Brain Tumor Pathol. 2010 Oct;27(2):95-101. Epub 2010 Nov 3. p53 abnormality and tumor invasion in patients with malignant astrocytoma.Momota H, Narita Y, Matsushita Y, Miyakita Y, Shibui S. Neurosurgery Division, National Cancer Center Hospital, 5-1-1, Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan. momota-nsu@umin.ac.jp AbstractMalignant astrocytomas are characterized by diffusely infiltrating nature, and the abnormality of p53 is a cytogenetic hallmark of astrocytic tumors. To elucidate the relationship between p53 abnormality and invasiveness of the tumors, we studied mutation and protein expression of p53 in 48 consecutive patients with malignant astrocytoma (14 anaplastic astrocytomas and 34 glioblastoma multiformes). The tumors were classified into three categories according to the features of magnetic resonance imaging, and 5, 7, and 36 tumors were classified into diffuse, multiple, and single type, respectively. We then examined how these tumor types correlate with MIB-1 staining index, TP53 gene mutation, and p53 protein expression. We found that p53 immunopositivity or TP53 mutation was frequently observed in diffuse and multiple types. These abnormalities of p53 were also associated with high MIB-1 staining index and strong expression of vascular endothelial growth factor. Furthermore, diffuse- and multiple-type tumors were significantly correlated with poor progression-free survival, whereas only multiple-type tumors were significantly correlated with poor overall survival. As diffuse and multiple features on imaging modalities represent invasive characteristics of the tumors, p53 abnormalities may affect the invasive and aggressive nature of malignant astrocytomas. |

- 王军臣
-
IHC标记出的P53蛋白一般来说是突变型P53基因的产物,因为野生型P53半衰期很短很短,不容易用IHC检测到。
P53的表达与胶质瘤细胞增殖活性相关,与胶质瘤分级、浸润、转移和预后相关。一般来说,高级别胶质瘤(WHOIII-IV级)例如胶质母多为弥漫强阳性表达,而低级别阳性率低,即便阳性也多为灶性表达。请见下文:
----
J Formos Med Assoc. 1999 Jan;98(1):31-8.
Expression and mutation analysis of the p53 gene in astrocytoma.
Hwang SL, Hong YR, Sy WD, Chai CY, Lin HJ, Howng SL.
Division of Neurosurgery, Kaohsiung Medical College, Taiwan.
Abstract
The role of p53 gene mutations in the formation or progression of human astrocytic tumors is controversial. We studied the distribution pattern of p53 immunoreactivity and analyzed p53 gene mutations to define the significance of p53 gene mutations in astrocytoma tumorigenesis or malignant progression. Twenty-three astrocytic tumors were evaluated with immunohistochemistry, single-strand conformation polymorphism (SSCP) analysis, and sequence analysis. We also searched MEDLINE to collect data on p53 gene mutation frequencies in astrocytic tumors in order to evaluate the association of p53 mutations and tumor grade. Strong immunoreactivity with a diffuse clustering pattern was found in three of five glioblastomas and seven of 12 anaplastic astrocytomas. Three of four low-grade astrocytomas were immunonegative. The p53 immunopositive cells in the only positively staining low-grade astrocytoma in our study appeared sparsely scattered. The results of immunostaining suggested that clonal expansion was associated with astrocytoma progression. Mutations of the p53 gene were detected in four of the 23 astrocytomas (one glioblastoma and three anaplastic astrocytomas). In the genetic data analysis, 76 of 367 astrocytomas had p53 gene mutations. A significantly greater p53 gene mutation frequency was found in anaplastic astrocytomas or glioblastomas than in the low-grade astrocytomas. The results of these immunohistochemical and genetic studies support the view that p53 gene mutation is associated with the malignant progression from low-grade to high-grade astrocytomas rather than with tumor initiation or promotion.

- 王军臣