| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- B2877乳腺肿块(20100914)--请看免疫组化图片!----浸润性乳头状癌??
| 姓 名: | ××× | 性别: | 女 | 年龄: | 47岁 |
| 标本名称: | |||||
| 简要病史: | |||||
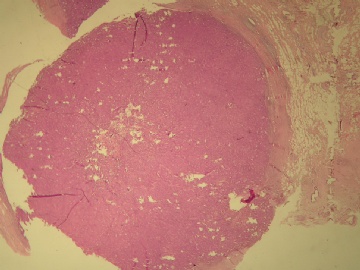
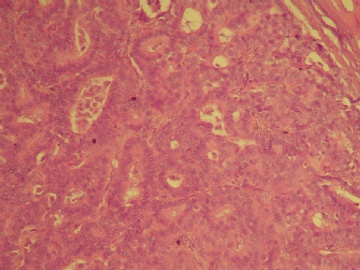
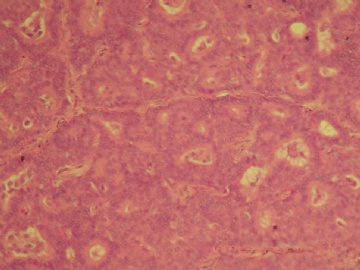
| 肉眼检查: | 乳腺肿块,大小1.2×1.0cm,边界清楚,质地中等。 | ||||
-
本帖最后由 于 2010-10-02 03:02:00 编辑
相关帖子
- • 乳腺肿瘤?
- • 右乳包块(镜下富于粘液)
- • 左乳肿块
- • 边界清楚的乳腺包块
- • 乳腺肿块,请会诊
- • 乳腺癌?
- • 左乳腺肿块,新加免疫组化
- • 左乳肿块,新加免疫组化
- • 乳腺包块-请会诊
- • 左乳肿块
-
本帖最后由 于 2010-09-29 19:47:00 编辑
This is our fellow and 同事's study. Till now it is best study in this area. EPC and SPC are very common lesions. Our surgeons and oncologists treat these lesions similar to DCIS. Patients will have segmental mastectomy. If the lesions are large, sentineal lymph node biopsy is considered. Till now we did not see any one sentineal lymph node positive case for these EPC and SPC without stroma invasion
Am J Clin Pathol. 2009 Feb;131(2):228-42.
Are encapsulated papillary carcinomas of the breast in situ or invasive? A basement membrane study of 27 cases.
Esposito NN, Dabbs DJ, Bhargava R.
Department of Pathology, Magee-Womens Hospital of the University of Pittsburgh, Pittsburgh, PA, USA.
Abstract
Encapsulated papillary carcinoma (EPC) of the breast is traditionally considered a variant of ductal carcinoma in situ (DCIS). However, recent studies show EPCs lack myoepithelial cells at their periphery, leading some to conclude that EPCs are invasive. We used a robust collagen type IV immunohistochemical procedure to assess invasion in 21 cases of pure EPC and 6 EPCs with adjacent invasive ductal carcinoma (IDC) and compared these results with those for papilloma, DCIS, and IDC. Moderate to intense collagen type IV expression was seen in all EPCs and was absent or decreased in all IDCs. All patients with pure EPC had negative axillary nodes with the exception of 1 who had a micrometastasis, and all were alive with no evidence of disease at follow-up (mean, 40.4 months). EPCs are in situ carcinomas with an excellent prognosis and can be managed with local therapy with or without sentinel lymph node biopsy.
-
本帖最后由 于 2010-09-29 19:47:00 编辑
Good review paper. find the full paper to read
Histopathology. 2008 Jan;52(1):20-9.
Papillary lesions of the breast: selected diagnostic and management issues.
Department of Pathology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02215, USA. lcollins@bidmc.harvard.edu
Abstract
The assessment and categorization of papillary lesions remains one of the most challenging areas in breast pathology. In this review, we will focus on several diagnostic and management issues related to papillary breast lesions that are frequently encountered in daily practice. These include: (i) the distinctions among papillomas with atypia (atypical papillomas), papillomas with ductal carcinoma in situ, and papillary ductal carcinoma in situ; (ii) recent developments in our understanding of encapsulated ('intracystic') papillary carcinomas and solid papillary carcinomas; and (iii) the impact of core needle biopsy on management decisions and specimen evaluation. The role of immunohistochemistry in the evaluation of these lesions, particularly the role of myoepithelial cell markers, will be emphasized.
-
本帖最后由 于 2010-09-29 19:48:00 编辑
Am J Surg Pathol. 2006 Aug;30(8):1002-7.
Intracystic papillary carcinomas of the breast: a reevaluation using a panel of myoepithelial cell markers.
Collins LC, Carlo VP, Hwang H, Barry TS, Gown AM, Schnitt SJ.
Department of Pathology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA 02215, USA. lcollins@bidmc.harvard.edu
Abstract
Intracystic papillary carcinomas (IPC) of the breast have traditionally been considered to be variants of ductal carcinoma in situ (DCIS). However, it is not clear if all lesions categorized histologically as IPC are truly in situ carcinomas, or if some such lesions might represent circumscribed or encapsulated nodules of invasive papillary carcinoma. Given that the demonstration of a myoepithelial cell (MEC) layer around nests of carcinoma cells is a useful means to distinguish in situ from invasive carcinomas of the breast in problematic cases, assessment of the presence or absence of a MEC layer at the periphery of the nodules that comprise these lesions could help resolve this issue. We studied the presence and distribution of MEC at the periphery of the nodules of 22 IPC and, for comparison, 15 benign intraductal papillomas using immunostaining for 5 highly sensitive markers that recognize various MEC components: smooth muscle myosin heavy chain, calponin, p63, CD10, and cytokeratin 5/6. All 22 lesions categorized as IPC showed complete absence of MEC at the periphery of the nodules with all 5 markers. In contrast, a MEC layer was detected around foci of conventional DCIS present adjacent to the nodules of IPC. Furthermore, all benign intraductal papillomas, including those of sizes comparable to those of IPC, showed a MEC layer around virtually the entire periphery of the lesion with all 5 MEC markers. In conclusion we could not detect a MEC layer at the periphery of the nodules of any of 22 lesions categorized histologically as IPC. One possible explanation for this observation is that these are in situ lesions in which the delimiting MEC layer has become markedly attenuated or altered with regard to expression of these antigens, perhaps due to their compression by the expansile growth of these lesions within a cystically dilated duct. Alternatively, it may be that at least some lesions that have been categorized as IPC using conventional histologic criteria actually represent circumscribed, encapsulated nodules of invasive papillary carcinoma. Regardless of whether these lesions are in situ or invasive carcinomas, available outcome data indicate that they seem to have an excellent prognosis with adequate local therapy alone. Therefore, we believe it is most prudent to continue to manage patients with these lesions as they are currently managed (ie, similar to patients with DCIS) and to avoid categorization of such lesions as frankly invasive papillary carcinomas. Given our observations, we favor the term "encapsulated papillary carcinoma" over "intracystic papillary carcinoma" for circumscribed nodules of papillary carcinoma surrounded by a fibrous capsule in which a peripheral layer of MEC is not identifiable.
-
本帖最后由 于 2010-09-29 19:48:00 编辑
This paper showed two cases of EPC with axillary lymph node micrometastasis. Now people think cases of EPC and SPC without frank stromal invasion ccannot have lymph node metastasis. If lymph node metastasis is present, the patients must have frank stromal invasion. For these two cases, most likely patients had stroma invasion and pathologists did not find becasue sampling issue.
Int J Surg Pathol. 2007 Apr;15(2):143-7.
Metastatic potential of encapsulated (intracystic) papillary carcinoma of the breast: a report of 2 cases with axillary lymph node micrometastases.
Department of Pathology and Laboratory Medicine, Mount Sinai Hospital, and the University of Toronto, Toronto, Ontario, Canada.
Abstract
Intracystic papillary carcinoma of the breast has rarely been reported to be associated with metastases. Presented are 2 cases, both of which showed micrometastatic carcinoma in axillary lymph nodes; neither demonstrated stromal invasion. Nearly complete absence of a myoepithelial cell layer around the periphery of the lesions was noted. One case showed 3 separate foci of micrometastatic carcinoma in 1 of 3 sentinel lymph nodes; the second showed micrometastases in 2 of 11 axillary lymph nodes. The clinical significance of micrometastases in lymph nodes associated with intracystic papillary carcinoma is unknown. The term encapsulated papillary carcinoma has been proposed to name papillary carcinomas that are surrounded by a fibrous rim, but which show a scant or absent myoepithelial cell component. The current 2 cases offer evidence to support the use of this term for particularly large encysted lesions. Sentinel lymph node biopsy may be prudent in such cases.
-
本帖最后由 于 2010-09-29 19:53:00 编辑
Thank Dr. Chiang to provide the good case and thank all people for attending the discussion. Internet is good. We do not have responsibility for the cases we discussed until they are your cases. (谢谢Dr. Chiang提供的好病例,也谢谢所有参与讨论的人。网络真好。除非是自己的病例,我们不需要对讨论意见负责。)
I think i have finished my discussion for this case and clearly showed my oppinion.信不信就由不得我了。ha.(我想我已经完成了对此例的讨论,已经完全表达了自己的观点。)
For some unclear issues, I suggest our pathologists to find some original papers to read. Remember that most text books are only summary or copy other's studies. (如果遇到不清楚的问题,我建议病理医生找到原文,直接阅读原文。记住,大多数教科书中仅仅是一些概括,或者复制了别的人研究观点)
"囊内乳头状癌(intracystic papillary ca)=包裹性乳头状癌(encapsulated papillary ca)"
1)在WHO的定义上“intracystic papillary ca”与`“encapsulated papillary ca”并不是同义词,不知我的理解是否正确?
2)WHO提及“乳腺浸润性乳头状癌、乳腺黏液性癌和乳腺髓样癌三者都是低度恶性的乳腺浸润性癌”,并把乳腺浸润性乳头状癌的形态限定在低级别IDC的组织学特征。
3)“encapsulated papillary ca”中的‘encapsulated’一词在甲状腺乳头状癌中译为“包膜型”,此处为“包裹型”,二者是否有区别?
4)对于encapsulated papillary carcinoma of breast的争论焦点-“无肌上皮细胞是否为浸润性癌”。 Dr. Zhao的同事用Laminin和IV型胶原IHC标记证明encapsulated papillary ca有基底膜,因此,认为是非浸润性癌。是否能理解为癌组织是被原导管的基底膜包绕?

- xljin8
-
本帖最后由 于 2010-09-29 20:03:00 编辑
This case bothers me and I cannot concentrate on my work. (这个病例使我烦恼,我无法专心工作)
EPC and 浸润性乳头状癌are two total different entities. The key of discussion for this case is not the nature of EPC or if EPCs have the trend for invasion. The key is the terminology we pathologists should give. (EPC和浸润性乳头状癌是两种完全不同的疾病实体。本例的关键讨论不是EPC的性质,也不是EPC的浸润潜能。关键是我们病理医生应该用哪个诊断名词)
As breast pathologist I have no doubt the diagnosis of 浸润性乳头状癌is totally wrong. It is not like a equevical case (ADH or DCIS). Suggest louzhu send out the case to get the second oppinion. Considering patient's treatment (partical mastectomy, total mastectomy, radiation, chemotherapy et al issue), pathologist should revise the surgical report, and communicate with surgeons, oncologists. Otherwise pathologists can get lawer suit for these kinds of cases.(作为乳腺病理医生,我坚信浸润性乳头状癌的诊断是完全错误的。它不像那种不确定性病例(ADH或DCIS)。建议楼主把病例送出会诊听听别人的意见。考虑患者的治疗(部分乳房切除、乳房全切、放疗、化疗等问题),病理医生应该修正报告,并与外科医生和肿瘤科医生交流。否则病理医生可能会因为这种病例而面临诉讼。)
Now I think I complete my duty as a pathologist without relation to the case.
-
本帖最后由 于 2010-09-29 20:04:00 编辑
I meant that I favor of the diagnostic suggestion by Dr.cqzhao, and I appriciated Dr. Chiang's warning.(我的意思是赞同Dr.cqzhao的诊断意见,也赞赏Dr. Chiang的提醒)
I know most EPCs are true variants of DCIS, as is uploaded by Dr. Chiang.
Dr. Xi Wang's case is DCIS-like invasion carcinoma.

华夏病理/粉蓝医疗
为基层医院病理科提供全面解决方案,
努力让人人享有便捷准确可靠的病理诊断服务。
 我很惶恐的回答金老师的两个提问,请金老师多多指教:
我很惶恐的回答金老师的两个提问,请金老师多多指教:
"囊内乳头状癌(intracystic papillary ca)=包裹性乳头状癌(encapsulated papillary ca)"?
WHO 2003应该更新了。Dr. Stuart J. Schnitt, M.D将是新版“WHO乳腺肿瘤分类”(第4版)的主编之一,我猜测新版中将会采取他的观点,如果他负责编写这一部分内容。
“encapsulated papillary ca”中的‘encapsulated’一词在甲状腺乳头状癌中译为“包膜型”,此处为“包裹型”?
这是翻译上的问题。
例句:Benign neoplasm: well circumscribed or encapsulated 良性肿瘤界限清楚或有包膜
对于良性肿瘤“encapsulated”确实应译为“有包膜的”;但作为某癌的定语,译为“包膜型”或“有包膜的”恐怕会令人误解此癌真有包膜?因此有意回避了“包膜”这个词。译为“包裹型”更能直观地体现此癌的一个特点,即癌周围包裹着厚厚的纤维性被膜。但愿国内同行能理解接收这种译法,更希望得到更多翻译高手指导,提出更好的译法。
迫切希望有关学术机制成立专业词汇委员会,制定专业词汇翻译标准。
谢谢金老师!

华夏病理/粉蓝医疗
为基层医院病理科提供全面解决方案,
努力让人人享有便捷准确可靠的病理诊断服务。
-
本帖最后由 于 2010-09-29 20:57:00 编辑
Abin老师太谦虚了,您对乳腺病理的功底和造诣是有目共睹!
在这里参与讨论的目的是为了澄清自己的一些模糊概念。我体会到在病理诊断的争议中,绝大多数是在定义、概念、和诊断标准上。尤其是少见的病种。
乳腺乳头状肿瘤可能是乳腺病理诊断中最难掌握的乳腺上皮性肿瘤之一,它包括乳头状瘤、不典型乳头状瘤、乳头状癌(又包括:原位,浸润,特殊亚型)。
楼主的诊断为“浸润性乳头状癌”,而Dr. Zhao认为“包裹性乳头状癌”, 并非常担忧“过诊断”会给病人带来“过渡治疗”,充分体现了一位优秀病理医生具备的素质。
我对包裹性乳头状癌并无经验,因此向大家请教如何掌握诊断标准。
9-11号听了王曦老师的在华夏病理网上的讲课,感触很大。一例“差分化浸润性导管癌”被诊断为“包裹性乳头状癌”, 而且经过美国顶级乳腺病理专家的会诊。由于诊断不足,手术后未能积极治疗,以致5个月后患者出现肝转移。教训真是太深刻了!
对于这个病例我感到首先要确定是不是乳头状癌。由于图片不够清楚,我无法辩别出有轴心的乳头,而是排列紧密的筛孔。于是很冒昧的提出了第一个问题:“无纤维血管轴心的乳头能否诊断为乳头状肿瘤?。

- xljin8
-
本帖最后由 于 2010-09-29 21:32:00 编辑
 金老师过奖,令我汗颜!
金老师过奖,令我汗颜!
我对乳腺病理的零星体会,主要来自学习网上病例。所以我要强力建议像我这样的初学者们,如果您在日常工作中的病例量不足(年检不到1万例)又想快速提高,上网学习是捷径!
我非常感激很多老师在网上深入探讨,非常感激老师朋友们提供了很多好病例!
金老师的提问,可以理解成学习指导。我得承认功力不足,暂时无法回答,不过我会针对性学习一下,找点资料看看。再次感谢!

华夏病理/粉蓝医疗
为基层医院病理科提供全面解决方案,
努力让人人享有便捷准确可靠的病理诊断服务。
-
xiaoxiulily 离线
- 帖子:26
- 粉蓝豆:265
- 经验:30
- 注册时间:2007-07-12
- 加关注 | 发消息
|
囊内乳头状癌的一个特点就是,肿瘤内和肿瘤周边都没有肌上皮存在。 CK5等高分子角蛋白在乳头状瘤常常阳性,而乳头状癌可以阴性。 |
| ------------------------------ |
| 博学之,审问之,慎思之,明辨之,笃行之。 |
|
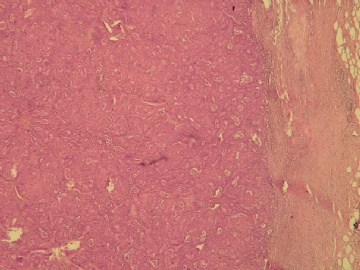
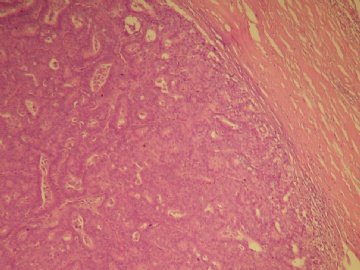
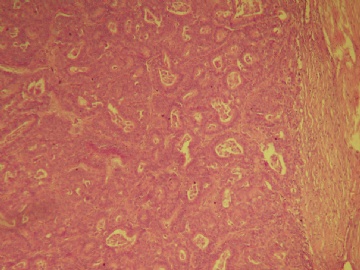
最后诊断:浸润性(实性)乳头状癌(infiltrating papillary carcinoma,IPC)。 IPC是一种少见的浸润性癌,结果类似非浸润性癌,如导管原位癌。该肿瘤特征是瘤细胞形成界限清楚的密集的乳头或实性团块,癌周与间质界限清楚,常有纤维增生,类似包膜或导管壁,HE染色多诊断导管内乳头状癌,而缺乏肌上皮则标志该肿瘤为浸润性癌。该例与同道分享的目的是:癌巢很圆滑或界限很明确的肿瘤可以是浸润性的,尽管少见,应标记肌上皮;同时表明,肿瘤的浸润方式不仅限于破坏性的,而也可见温和性的膨胀型或推挤型浸润。类似的病变还有浸润性粉刺样癌,后者HE类似粉刺型导管原位癌,但癌巢周缺乏肌上皮,尽管少见,工作中当想到有这类病变的可能,并进一步做些工作,会使诊断更恰当。 |
学习中。。。。。。