| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
-
关于癌前病变、宫颈癌及HPV的关系

| 性别 | 女 | 年龄 | 0 |
|---|---|---|---|
| 一般病史 | 0 | ||
| 标本类型 | 0 | ||
| 制片方法 | 0 | ||
| 染色方法 | 0 | ||
关于癌前病变、宫颈癌及HPV的关系
今天在罗氏网站中看到了这样一段话,有很大的疑问,不知哪位大神能解答下,以下是原文:
HPV是一种极为常见的病毒,高达75%的女性在一生的某一阶段会感染HPV。HPV持续感染是宫颈癌及其癌前病变——宫颈上皮内瘤变(CIN)——的主要病因,且宫颈病变程度越重,高危HPV 感染率越高。在CIN 1、CIN 2 、CIN 3患者中,HPV感染率分别为30 %、55 %和65 %,而99.8%以上的宫颈癌患者体内能检测到HPV感染,HPV阴性者几乎不会发生宫颈癌2,3。
最新的cobas HPV基因检测,则检测宫颈位置是否存有HPV,并不需要主观分析或诠释,具有更高的灵敏度和可重复性,已被美国临床广泛接受。ATHENA研究发现,在年龄30岁及以上的妇女中,每10位HPV 16和/或18阳性的女性中就有1位为宫颈癌前病变,而她们的巴氏涂片检查结果是正常的。此外,许多文献报道发现,单纯使用HPV基因检测CIN2或CIN3的灵敏度达94.6%,而细胞学方法的灵敏度仅有一半左右,但两者结合的灵敏度可以达到100%9 。
大家请注意看大字黑体中的数据,我的疑问是:CIN2、CIN3的HPV感染系分别为30%、55%和65%,文中却说使用HPV检测CIN2或CIN3的灵敏度却为94.6%,大家觉得有没有问题。
我猜大家可能会提出HPV感染率与灵敏度是两个概念,两个是没必然的联系的。这就关系到灵敏度的定义了,在HPV基因检测中,灵敏度是否指能检测HPV基因的概率呢?但CIN2中HPV感染率为55%,为何HPV基因试剂能检测94.6呢?举个例子:100例,CIN2患者中,有55位有HPV感染,但HPV基因检测却为94位。
哪位老师能为我解答下,谢谢!
巴山夜雨涨秋池: glad to meet you here.
I have a review article about cervical cancers with negative HPV testing,which should be published in 中华病理学杂志第一期2015. You can find to read if you are interested. Or can you list the paper here if you can find. thanks, chengquan
可是最新的说法是诊断CIN必须有HPV感染啊,看不见HPV的感染的证据就不能报CIN。不知道对不对啊?请老师告诉俺好吗?
Not sure you talk about cytology or histology.
Part of what you said is right and part is not correct.
典型的挖空细胞 in the pap means LSIL or CIN1 in histology which is the evidence of HPV infection. But for HSIL in Pap or CIN2/3 or cervical cancer, you make diagosis based on the cytology or morphology. However you cannot make diagosis of HPV infection based on the cytomorphology, although most of them are HPV infection related
感谢赵老师光临指导。
我在这里自言自语一段话,无任何针对性,即不针对楼主,也不针对赵老师。
感染性疾病检测:抗原,抗体,DNA-RNA或“基因检测”。
抗原阳性:有病原体的成份。有病原体的成份,就一定有活的病原体吗?不一定。比如乙肝表面抗原阳性,能说有活的乙肝病毒吗?不能。
抗体阳性:有病原体感染后机体反应生成的“标志”。有反应标志,就一定有活的病原体吗?不一定。比如 1 型疱疹病毒IGG阳性,能说有活的 1 型疱疹病毒吗?不能。
DNA阳性:有病原体DNA片断。有DNA片断,就一定有活的病原体吗?不一定。DNA只是病原体具备遗传信息的一部分,并不是完整的病原体。
病原体是否存活,对感染性疾病的转归或愈后无疑是重要的,但谁知道活不活呢?
宫颈癌病例,有一部份毫无疑问HPV是阴性的。这不是试剂或方法敏感性不够的问题。不必期望宫颈癌病例100%检出HPV。用辨证和发展的眼光看问题,可以理解这一结论。多年的HPV持续感染,已导致组织病变 / 癌变,而机体的变化,可能改变其曾经的免疫耐受状态,从而清除HPV病毒,但生米已成熟饭。。。。。。。。
欢迎有空的朋友继续唠嗑儿。

body>h1>span>...................This signature is very handsome.
Human Papillomavirus Testing and Reporting Rates in 2012: Results of a College of American Pathologists' National Survey.
Abstract
最新的cobas HPV基因检测,则检测宫颈位置是否存有HPV,并不需要主观分析或诠释,具有更高的灵敏度和可重复性,已被美国临床广泛接受。
It is true that FDA approved cobas HPV can be used as primary screening for cervical cancer in April 2014. But in term of my knowledge, HPV as primary screening has not be used by any hospital in the USA.
- Wright TC Jr., Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206:46.e1–46.e11.
- Sasieni P. Estimating prevalence when the true disease status is incompletely ascertained. Stat Med. 2001;20:935–949.
- http://j.mp/stoler-thomas-athena
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007; 357:1579–1588.
- Stoler MH, Wright TC Jr., Sharma A, et al. The interplay of age stratification and HPV testing on the predictive value of ASC-US cytology. Results from the ATHENA HPV study. Am J Clin Pathol. 2012;137:295–303.
- http://j.mp/ncsp-renewal
CAP Today Home » 2014 Issues, November 2014, Regular features
Letters (November 2014)
HPV primary screening
We read with great interest the two recent articles by William Check, PhD, highlighting primary HPV testing proposals (June and September 2014). Additional related information not covered in the two CAP TODAY articles should be brought to readers’ attention.
The first article focused on the ATHENA trial, in which a primary human papillomavirus screening algorithm based on the Roche Cobas HPV assay was evaluated. Although cervical cancer risk increases significantly with age (Cancer. 2014;120:2032–2038), no mention was made of ATHENA trial data documenting that the proposed Cobas primary HPV screening algorithm had not only limited overall verification-bias–adjusted sensitivity for detection of cervical intraepithelial neoplasia 3 (CIN3) and cervical cancer (CIN3+) but that this limited sensitivity steadily decreased even further among the highest risk older age groups (Table 1). The low documented rates for CIN3+ detection (27 to 58 percent) with the proposed Cobas algorithm intuitively raise questions about the safety of the algorithm and its use with extended screening intervals.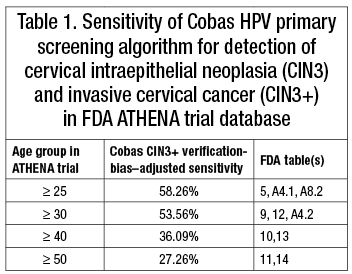
The second CAP TODAY article focused on a recent publication analyzing risk for CIN3 and cervical cancer during a lengthy experience with conventional Pap and Hybrid Capture 2 (HC2) HPV cotesting in the Kaiser Permanente Northern California system (Gage JC, et al. J Natl Cancer Inst. 2014;106[8]:pii:dju153). While lead author Julia C. Gage, PhD, MPH, acknowledges in the CAP TODAY article that the statistically significant lowest risks for CIN2 and CIN3 were achieved with Pap and HC2 HPV cotesting, the difference in risk between cotest-negative and HPV-negative patients is dismissed as “small.” The CAP TODAY article goes on to add: “The difference in cancer risk was small and not statistically significant.” Although the JNCI authors have chosen thus far not to allow independent statistical evaluation of their privately held data set or to disclose most P values in the data comparisons,
Dr. Gage clearly said in a public presentation in August at the 29th International Papillomavirus meeting in Seattle that the lowest cancer risk at three years after negative cotest results (0.007 percent) was “statistically significant” when compared with cancer risk after a negative HC2 HPV test (0.011 percent). The P value comparing the lower cancer risk at five years after negative cotest results (0.014 percent) with cancer risks after negative HC2 HPV test results (0.017 percent) is still undisclosed.
When the Pap test was introduced after World War II, the emphasis was on decreasing cervical cancer morbidity and mortality. Later, as new cervical screening technologies emerged in the late 1990s in response to observations on the limitations of screening, the Agency for Health Care Policy and Research noted that the verification-bias–adjusted sensitivity of the conventional Pap smear was only “near 50 percent, much less than generally believed” (AHCPR, Evaluation of Cervical Cytology, 1999), and that decreased cancer incidence and mortality would require some combination of increased recruitment to screening, increased screening test sensitivity, and more frequent screening. Available SEER data through 2011 documents that cervical cancer rates have continued to decline in the new screening technology era. As the new era of screening using HPV testing continues to develop and evolve, it is surprising to observe that a new proposed HPV primary screening algorithm with FDA-documented CIN3+ sensitivity “near 50 percent” is being portrayed as a significant advance and seen as the basis for a shift from a continued focus on further reduction of cervical cancer through screening to a new emphasis on 1) extending screening intervals, 2) achieving only “reasonable” cervical cancer risk (now apparently defined as risk associated with every three-year screening with the conventional Pap test), and 3) decreased testing and associated health care expenditures. As Dr. Gage said somewhat dismissively in Seattle, “You can always get a slightly lower risk of cervical cancer by reducing your interval or adding additional tests.”
R. Marshall Austin, MD, PhD
Chengquan Zhao, MD
Department of Pathology
Gynecologic Pathology Division
Magee-Womens Hospital of University of Pittsburgh Medical Center
■ Mark Stoler, MD, cytopathologist and professor emeritus of pathology and clinical gynecology, University of Virginia, replies: I thank Dr. Austin and Dr. Zhao for their ongoing interest in the comparison between the potential of an HPV primary screening algorithm and cotesting. In my opinion, CAP TODAY and especially its reporter William Check, PhD, have done a superb job in presenting both sides of the argument in a balanced manner. In response to some of Dr. Austin and Dr. Zhao’s specific comments, I would briefly add the following:
Verification bias adjustments, where any detectable disease in a sample of patients with normal screening tests is then extrapolated to the whole population, always have a larger impact on apparent sensitivity than they do specificity. In the opinion of many epidemiologists, this impact is now understood to potentially be very misleading, but in general the relative rankings of the tests do not change.1,2 Thus in ATHENA, while the verification-bias–adjusted sensitivities “sound” low, the VBA sensitivity of cytology is lower than for HPV in all comparisons. Furthermore, as was pointed out in a prior online exchange,3 the figures quoted in the table in Dr. Austin’s letter are the VBA sensitivity of the algorithm, not the test. Unfortunately, individuals often confuse VBA and non-VBA statistics from different sources and compare apples to oranges, including Drs. Austin and Zhao. In numerous published studies, the unadjusted sensitivity of cytology is on the order of 50 to 60 percent, whereas the unadjusted sensitivity of a clinically validated HPV test is always on the order of 90 to 95 percent. As noted in reference No. 3, the published unadjusted sensitivity of the Cobas HPV test for ≥ CIN3 is 92.0 percent compared with 75.1 percent for liquid-based cytology. The performance of both HPV testing and cytology in ATHENA is similar to what was found in the only other large North American screening study, Mayrand, et al., who reported an unadjusted sensitivity of Hybrid Capture 2 of 82.8 percent and 57.7 percent for cytology for ≥ CIN 2.4
The decrease in sensitivity with age of all cervical cancer screening tests is not a new phenomenon, nor perhaps an unexpected one given the known regression of transition zone up the canal inducing sampling issues confounded by the known problem pathologists have with age-related mimics of HSIL, an issue we discussed in reference No. 5. Still, in head-to-head, apples-to-apples comparisons like ATHENA, HPV testing is superior to cytology in sensitivity with only minimal impact on specificity across all age groups. This fact, together with the data presented to and independently analyzed both by the FDA advisory panel and yet again by the FDA, strongly supports our belief that the time has come to consider seriously the benefits and simplicity of an HPV-based primary screening algorithm for cervical cancer screening. Such an approach is gaining worldwide acceptance and will become increasingly necessary as one considers the need to screen populations that have been vaccinated against HPV, where the performance of cytology will only degrade further.6 I am sure we can all agree that the United States should “only have the problem” of having to address how to screen a properly vaccinated and protected population.
■ Julia C. Gage, PhD, MPH, research fellow, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, and Thomas S. Lorey, MD, medical director of TPMG Regional Reference Laboratory, Kaiser Permanente Northern California Region, reply:
We have some clarifications in response to the letter written by Drs. Austin and Zhao.
If a study is large enough, even small differences between very low risks can be statistically significant as seen in our findings from KPNC. When comparing the cancer risks after an HPV-negative result with cancer risks after a cotest-negative result at the same time point (i.e. three or five years), the risks have overlapping 95 percent confidence intervals. The three-year cancer risk after an HPV-negative versus cotest-negative result is statistically significant (0.011 versus 0.007, P=.03) while the five-year cancer risk after an HPV-negative versus cotest-negative result is not (0.017 versus 0.014, P=.11).
Importantly, statistical significance should not be conflated with identifying the optimal screening strategy. The decision of how often to screen with what tests is increasingly more complicated and there will always be opportunities to push further toward greater reassurance by using more screening tests or shortening intervals or both. At an extreme, women could be screened every six months with cotesting, but we all realize this would result in excessive identification of benign transient infections and associated changes. A balance must be struck between greater reassurance and the associated costs and harms. We turn to professional guidelines committees, which are best suited to such an endeavor.
Use HPV test in conjunction with Pap
Published data show that an HPV test can fail to detect nine percent to 20 percent of cervical cancers (Zhao C, et al. Evidence emerging for HPV-negative cervical cancer. CAP TODAY, January 2014). At BioReference Laboratories, a national clinical laboratory with specialized expertise in women’s health, cancer, and genetics, we examined relevant data over a one-year period. Our review disclosed that 66 of 733 (nine percent) Pap tests interpreted as suspicious for cancer (high-grade lesions, or HSIL) had a negative Roche Cobas HPV test. If these women had been tested only with the Roche Cobas HPV assay, their potentially precancerous condition (HSIL) would have been missed. In addition, a number of the women who had HSIL Pap results accompanied by a negative Roche Cobas HPV test had the more definitive test for cancer—a cervical biopsy. Of the abnormal biopsies, 10 percent showed squamous cell carcinoma of the cervix, 40 percent showed other precancerous high-grade lesions (CIN 2/CIN 3), and 50 percent had endometrial cancer. These cancers were detected based on a positive Pap test.
In these times of rising health care costs where findings based on population studies may change recommended medical practice guidelines for individual patients, any such changes must be fully vetted and appropriately balanced before they are implemented. For this reason we strongly believe that rather than replace the Pap test, the HPV test should be used in conjunction with the Pap test to assess a woman’s true risk of cervical cancer. While we welcome new advances in medicine, we should learn from previous data and gather additional information before changing our cervical cancer screening behaviors. In light of Pap testing having been enormously successful in reducing deaths from cervical cancer and the cervical cancer screening guidelines having been changed as recently as 2012, we believe more caution and research are warranted before additional changes are made to a screening algorithm.
James Weisberger, MD
Chief Medical Officer
James W. Sharp, MD
Clinical and Anatomical Pathologist
Vassar Brothers Medical Center
Jeffrey Gilbert, MD
Medical Director, STIs
Frank Buccini, MS
Director of Molecular Diagnostics
BioReference Laboratories
Elmwood Park, NJ
Genomic testing and the LIS
In “Why LIS limitations shouldn’t inhibit genomic testing” (Newsbytes, September 2014), Lynn Bry, MD, PhD, said “outside of the upfront sample handling and getting results out the door and billing for it, you probably aren’t going to be able to handle the intermediate steps with current lab information systems.” This continues to be true for many vendors, but in December 2013 Sunquest Information Systems released a complex testing workflow application, completely integrated with the core laboratory information system, that supports genotyping and is well suited to handle the wet bench work of sequencing applications.
This year, Sunquest made a strategic investment in GeneInsight, an IT platform company that streamlines the analysis, interpretation, and reporting of complex genetic test results. Sunquest and GeneInsight are teaming up to offer clients a complete genetics workflow, with integration enabling the exchange of structured information.
It is the case that “Some companies have developed laboratory information management systems to support complex sequencing in a research setting, ‘but what they often lack is the capacity to operate effectively in a CLIA laboratory.’” Sunquest and GeneInsight have been operating in a clinical setting for years and are both registered class I exempt medical devices. Let Sunquest be the first to say, “Here’s a solution that’s going to help you with your complex genomic testing.”
Megan Schmidt
Director, Product Strategy
Sunquest Information Systems
■ Lynn Bry, MD, PhD, medical director and associate pathologist at Brigham and Women’s Hospital, Harvard Medical School, replies: BWH is a founding institution of Partners Healthcare, a majority shareholder in the GeneInsight application. I was aware of the potential partnership between GeneInsight and Sunquest but unable to discuss it at the Pathology Informatics 2014 conference, and later when interviewed by CAP TODAY, as the agreement was still under negotiation. The features offered by GeneInsight cover variant calling from processed sequences, curation of content for evaluating the variants, generation of reports, and data transmission to other clinical systems. Integrating these and earlier stages of bioinformatic processing with clinical information systems will further support genomic analyses in clinical laboratories.
TRALI
Your article on TRALI (October 2014) reported that the BloodCenter of Wisconsin is one of a few laboratories that do most of the human neutrophil antigen testing through their own laboratory-developed tests. As the manager of one of the laboratories that does this HNA testing, I was surprised you named only the BloodCenter of Wisconsin. Our laboratory at the American Red Cross deserved mention also for the following reasons, among others.
The Neutrophil Laboratory at the American Red Cross was developed by Jeffrey McCullough, MD, in 1985. Dr. McCullough along with Dave Stroncek, MD, also a former laboratory director, have been world leaders in the research of neutrophil testing and TRALI investigations for decades. Dr. McCullough was the primary author of Granulocyte Serology: A Clinical and Laboratory Guide (ASCP Press, 1988), which is still used today as a source for laboratory methods linked with HNA laboratory analysis.
Our laboratory and the lab at the BloodCenter of Wisconsin are the only two neutrophil laboratory members in the U.S. associated with the International Society of Blood Transfusion Working Party on Granulocyte Immunobiology. Both of the labs participate in the quality assurance exercises distributed by the International Granulocyte Immunology Workshops (IGIW). The American Red Cross Neutrophil Laboratory is one of four reference laboratories in the IGIW that organize and distribute these QA samples. (The others are in Bristol, U.K., Hagen, Germany, and Amsterdam, the Netherlands.)
The IGIW has recommended a combination of the granulocyte immunofluorescence test (GIFT) and the granulocyte agglutination test (GAT) when detecting and identifying HNA antibodies (Transfusion. 1997;37:977–983; Transfusion. 2002;42:462–468; Vox Sang. 2013;105:259–269). The American Red Cross is the only neutrophil lab in the U.S. that routinely tests all donors and patients for both GAT and GIFT. All other neutrophil laboratories in the U.S. routinely screen samples for HNA antibody by GIFT.
Your article referred to the four-year prospective study led by Pearl Toy, MD, as the seminal investigation in TRALI (Blood. 2012;119[7]:1757–1767). Dr. Toy named our laboratory the neutrophil laboratory to perform all HNA antibody testing for this National Heart, Lung and Blood Institute
SCCOR study.
Randy M. Schuller
Neutrophil and Platelet
Immunology Laboratory Manager
Mid-America Blood Services Division
American Red Cross
Saint Paul, Minn.
Human Papillomavirus testing and cytologic/histopathologic “test of cure” follow-up results after excisional treatment for high grade cervical intraepithelial neoplasia
Is 58% sensitivity for detection of cervical intraepithelial neoplasia 3 and invasive cervical cancer optimal for cervical screening?
Abstract
Prior high-risk human papillomavirus testing and papanicolaou test results of 70 invasive cervical carcinomas diagnosed in 2012: results of a retrospective multicenter study.
Abstract


 。。。。都是些“鸡肠”翻译得好累啊,
。。。。都是些“鸡肠”翻译得好累啊,















