| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- B3087有难度的乳头状肿瘤病例(非典型性周围型导管内乳头状瘤还是周围型导管内乳头状瘤伴DCIS?)新加病灶测..
| 姓 名: | ××× | 性别: | F | 年龄: | 37 |
| 标本名称: | |||||
| 简要病史: | |||||
| 肉眼检查: | |||||
病历摘要:
“发现左乳肿物1年余,右乳肿物1周”入院。
2010年12月6日双乳钼靶片示:双乳呈混合型Ⅳb(纤维囊性增生为主),左乳外上区多发肿块不除外恶变可能,建议手术,右乳外上区良性增生结节,BI-RADS:III,左乳BI-RADS:V;
查乳腺彩超示:符合双侧乳腺增生声像,左乳头外上象限低回声团块,考虑为实性占位,不除外恶变可能,建议病理检查,右乳低回声肿块,考虑纤维腺瘤可能。
专科检查:双乳外形欠对称,左乳12-2点位距乳头2cm可触及一肿物,大小约4.0×5.0cm,质硬,边界欠清,表面欠光滑,活动度欠佳,与皮肤、胸壁无粘连。右乳11点位距乳头2cm可触及一肿物,大小约1.5×1.5cm,质韧,边界尚清,表面尚光滑,活动度一般,与皮肤、胸壁无粘连。
临床诊断:
1.乳房肿物(左乳癌?)2.乳房肿物(右乳增生结节?)3.乳腺增生(双侧)
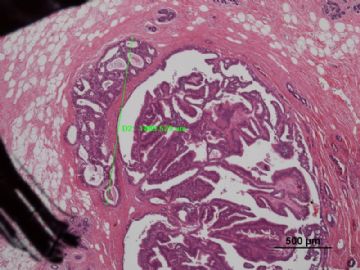
大体病理检查:
送检(左侧)乳腺肿物切除标本:6.0cm×4.0cm×1.0cm,切面见大小为1.5cm×1.3cm灰红结节。
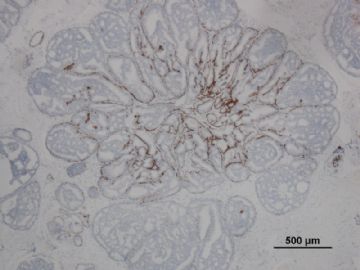
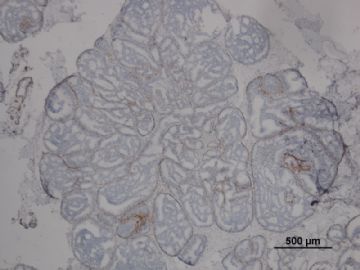
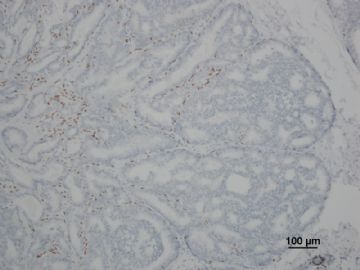
其中一张切片,免疫组化顺序为:ER,CK5/6,CD10
-
本帖最后由 于 2011-01-07 21:28:00 编辑
相关帖子
- • 乳腺包块
- • 左乳癌标本乳头一个导管内的病变
- • 乳腺两个相邻导管内的病变
- • 乳腺肿物
- • 乳腺肿物,请各位老师帮忙会诊
- • 女 46岁发现左乳腺肿块一月余
- • 乳腺包块。33岁
- • 左乳肿块,协助诊断
- • 乳腺肿物
- • 乳腺肿物
-
本帖最后由 于 2011-01-07 12:00:00 编辑
引用一些诊断标准:
区分不典型乳头状瘤和乳头状瘤伴DCIS的标准
区分不典型乳头状瘤和乳头状瘤伴DCIS的标准不统一。
一些专家将含有局限区域非高级别DCIS的乳头状瘤(例如,DCIS范围<3mm或DCIS占病变的比例<30%)定义为“乳头状瘤伴非典型性“或”非典型乳头状瘤”,而当DCIS较大或占病变更多比例时则诊断为乳头状瘤伴DCIS。
另有人提出当乳头状瘤的非典型增生区域具有DCIS的结构和细胞学特征时,不论其范围如何,均诊断为乳头状瘤伴DCIS。
-Stuart J,et al(黄文斌等译).乳腺病理活检解读,P207.
ADH和DCIS的量化鉴别
<非典型性导管增生章节的论述>:
1、将一个或一个以上完全累及的导管 横切面合计≤2mm规定为ADH上限;
2、另一些学者则要求两个区域具有典型的细胞形态和结构:①增生细胞单一性,②核/浆比增大;③细胞核等距或极规则分布;④圆形核;⑤可出现或不出现染色质浓染;⑥拱形、筛状、实性和/或微乳头型。
<低级别导管内癌章节的论述>:
低级别DCIS由小的单一性细胞组成,生长方式呈拱形、微乳头、筛状或实性,核大小一致,染色质均匀,核仁不明显,核分裂象罕见。
1、某些诊断标准要求单个导管横切面全部被特征性细胞和结构替代,
2、而有些则要求2个管腔或者1个以上导管横切面直径达到2mm。
-Tavassoli FA,et al.乳腺及女性生殖器官肿瘤病理学和遗传学.
复习以上标准:DCIS及乳头状瘤合并DCIS与ADH的诊断分歧主要是病变的“质”和“量”是否达到DCIS诊断标准的问题。
本例“质”是否达标?“量”是否达标?
大家在日常工作中是如何把握以上标准的?
请大家继续发表意见,谢谢!
-
本帖最后由 于 2011-01-09 21:57:00 编辑
Lewis JT, Hartmann LC, Vierkant RA, Maloney SD, Shane Pankratz V, Allers TM,Frost MH, Visscher DW. An analysis of breast cancer risk in women with single, multiple, and atypical papilloma.
Am J Surg Pathol. 2006 Jun;30(6):665-72.
Division of Anatomic Pathology, Mayo Clinic, Rochester, MN 55905, USA. lewis.jason@mayo.edu
Breast papillomas may be single or multiple and associated with atypical ductal or lobular hyperplasias (ADH/ALH). The risk of breast carcinoma development in patients with papillomas, particularly those with multiple or atypical lesions,is incompletely defined. Fibrocystic lesions were histopathologically classified in a benign breast disease cohort of 9155 who underwent biopsy from 1967 to 1991,with papilloma assessment in 9108.
Individuals with papillomas (N=480) were classified into 4 groups:
1)single papilloma (SP, N=372),
2)single papilloma with ADH or ALH (SP+A, N=54),
3)multiple (>5) papillomas (MP, N=41),
4)multiple papillomas with ADH or ALH (MP+A, N=13).
Those without papillomas were classified as nonproliferative (NP, N=6053), proliferative without atypia (PDWA, N=2308), and ADH/ALH [atypical hyperplasia (AH), N=267]. The relative risk of cancer development within our cohort was compared to that expected in the general population using standardized incidence ratios.
The relative risk of breast cancer development associated with SP [2.04, 95% confidence interval (CI)1.43-2.81] was greater than NP (1.28, 95% CI 1.16-1.42) but similar to PDWA (1.90, 95% CI 1.66-2.16).
The risk associated with SP+A (5.11, 95% CI 2.64-8.92) was highly elevated but not substantively different than atypical hyperplasia (4.17, 95% CI 3.10-5.50).
Patients with MP are at increased risk compared with PDWA or SP (3.01, 95% CI 1.10-6.55), particularly those with MP+A (7.01, 95% CI 1.91-17.97). There was a marginal increase in breast cancer risk (16%) among patients with proliferative disease if a papilloma was present, but this did not reach statistical significance (P=0.29).
The observed frequency of ipsilateral(vs. contralateral) breast cancer development in papilloma subsets was not significantly different than other patient groups.
We conclude that:
1)SP imparts a cancer risk similar to conventional proliferative fibrocystic change. The presence of papilloma in, or associated with, atypia does not modify the risk connotation of ADH/ALH overall.
2)MP constitutes a proliferative breast disease subset having unique clinical and biologic behavior.
这篇文献研究了乳腺乳头状瘤伴或不伴不典型导管或小叶增生、多发性乳头状瘤伴或不伴不典型导管或小叶增生与非乳头状乳腺增生或不典型增生发生乳腺癌的危险性。红笔字处讲的:多发性乳头状瘤的危险性为3.01; 多发性乳头状瘤伴ADH/ALH的危险性为7.01。明显高于其他组。这说明了鉴别诊断的临床意义。

- xljin8
-
本帖最后由 于 2011-01-22 06:48:00 编辑
| 以下是引用老山羊在2011-1-6 12:11:00的发言: 引用一些诊断标准:
区分不典型乳头状瘤和乳头状瘤伴DCIS的标准 区分不典型乳头状瘤和乳头状瘤伴DCIS的标准不统一。
ADH和DCIS的量化鉴别 <非典型性导管增生章节的论述>: 1、将一个或一个以上完全累及的导管 横切面合计≤2mm规定为ADH上限; <低级别导管内癌章节的论述>: 低级别DCIS由小的单一性细胞组成,生长方式呈拱形、微乳头、筛状或实性,核大小一致,染色质均匀,核仁不明显,核分裂象罕见。 1、某些诊断标准要求单个导管横切面全部被特征性细胞和结构替代, 2、而有些则要求2个管腔或者1个以上导管横切面直径达到2mm。
复习以上标准:DCIS及乳头状瘤合并DCIS与ADH的诊断分歧主要是病变的“质”和“量”是否达到DCIS诊断标准的问题。 本例“质”是否达标?“量”是否达标? 大家在日常工作中是如何把握以上标准的? 请大家继续发表意见,谢谢! |
感谢楼主提供的鉴别诊断标准。
如何理解和运用?
1)乳腺的乳头状病变是一组不同性质的导管上皮增生性病变,以形成具有纤维血管轴心的乳头为其共同的形态学特征;
2)乳头状瘤-不典型乳头状瘤-乳头状原位癌三者之间并无我们了解的结直肠“腺瘤-癌”的关系(至少目前并未证明)。
3)不典型乳头状瘤(乳头状瘤伴ADH)的病理诊断标准至今并无共识。复习“乳腺病理活检解读”204-207页,8.2 乳头状瘤伴非典型性(非典型乳头状瘤)和乳头状瘤伴导管原位癌一节, 您会看到上述不同的观点。Page(1996)要求乳头状瘤中形态具有DCIS改变的病灶直径大于3mm。Tavasoli(1999)要求DCIS大于30%。Elston(1998)认为乳头状瘤中不典型区域只要具有DCIS的结构和细胞学特征时,无论面积大小,都要诊断为“乳头状瘤伴DCIS。 Dr.Schnitt同意Elston的观点。 Dr.Schnitt在对乳头状瘤伴DCIS的形态学描述中讲到"乳头状瘤中的导管原位癌往往表现为实体或筛孔状结构,细胞核为低级别或中级别,可见小灶性坏死"。
4)参阅Tavasoli(2009年)主编的AFIP“Tumors of the mammary gland", 她把乳头状肿瘤分为:乳头状瘤、不典型乳头状瘤[分二种情况:1)乳头状瘤中出现上皮细胞复层和肌上皮细胞消失区域;2)乳头状瘤中出现实体、筛孔或微乳头的低级别DCIS区域,范围小于整个病变的30%]、DCIS来源于乳头状瘤(低级别DCIS的范围可占病变30%-90%)、乳头状导管内癌。
5)在实际工作中,中央性乳头状瘤与乳头状导管原位癌的的鉴别诊断一般比较容易。但是,周围型乳头状瘤伴ADH与周围型乳头状瘤伴DCIS的鉴别诊断比较困难。诊断时要注意:1)乳头状瘤的周围组织中是否存在典型的低-中级别DCIS;2)如DCIS病灶仅出现在乳头状瘤中,要仔细区分病变的不同性质,究竟是不典型乳头状瘤?是DCIS?还是乳头状瘤伴DCIS?
6)运用肌上皮细胞消失作为鉴别点时要注意:乳头状瘤伴DCIS时仅在乳头状瘤中的DCIS区域肌上皮消失,而不是整个乳头状瘤中肌上皮完全消失, 残留的乳头状瘤区域肌上皮可以存在(参照“乳腺病理活检解读”,206页,图8.13)。 分清楚这一点对于鉴别诊断尤为重要!
7)此外鉴别乳头状瘤中DCIS病灶时,高分子量角蛋白(CK5/6和34BE12)的失表达(图8.14)和ER均一性表达也是非常有价值的辅助指标。
8)应该认为诊断DCIS只有一个形态学标准,它即可用于DCIS与ADH的鉴别,也适用于乳头状瘤伴ADH与乳头状瘤伴DCIS的鉴别。
9)鉴别乳头状瘤伴ADH和乳头状瘤伴DCIS的临床意义二种不同程度的病变发生乳腺浸润性癌的危险性不同,而乳头状瘤伴DCIS的危险程度又与DCIS病灶的大小呈正相关。
再次感谢楼主的严谨态度和一丝不苟!
上述为个人学习的体会,仅供参考和交流,错误难免,请指正。
谢谢!

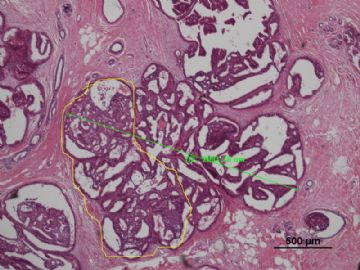
名称:图1
描述:图1

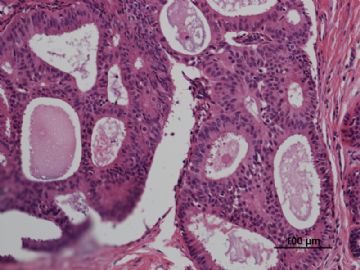
名称:图2
描述:图2

- xljin8
-
本帖最后由 于 2011-03-20 23:39:00 编辑
Papillomas can display focal proliferations of a mildly
atypical, monotonous cell population identical to lowgrade
DIN (DIN 1/ADH/DCIS, grade 1; Figures 5, A
through F, and 6, A through C). When DIN 1 occupies
less than a third of the papillary lesion, the term atypical
papilloma has been used. If at least a third, but less than
90% of the lesion displays such changes, the designation
of carcinoma arising in a papilloma has been used.
When completely excised, these 2 groups do not seem to differ in clinical behavior, as observed in a retrospective study that used this quantitative approach. It is noteworthy that
although 90% was used as the cut-off point, none of the
lesions had atypical areas that occupied more than 65%
to 70% of the papillary lesion. Currently, to avoid the term
carcinoma, we designate all such lesions as papillomas with
DIN 1/atypical papilloma (Table 1).
-Ueng, S.H., T. Mezzetti, and F.A. Tavassoli, Papillary neoplasms of the breast: a review. Arch Pathol Lab Med, 2009. 133(6): p. 893-907.

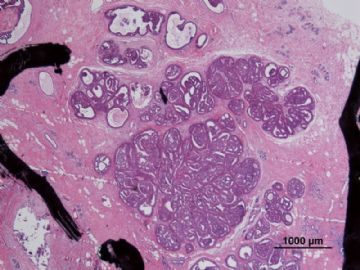
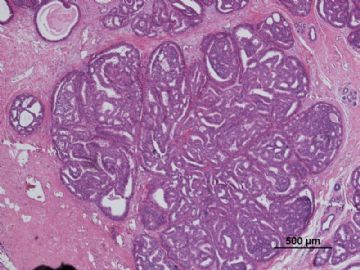
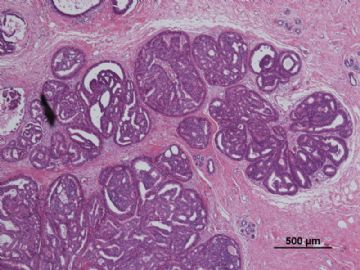
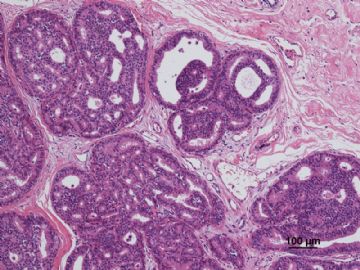
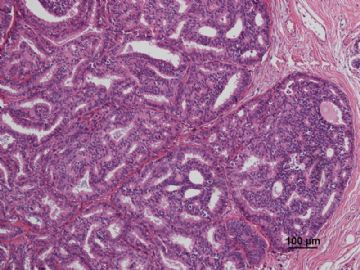
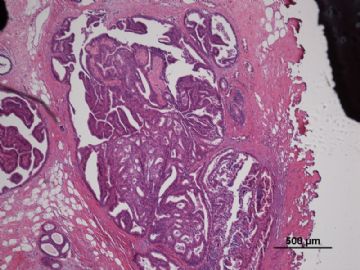
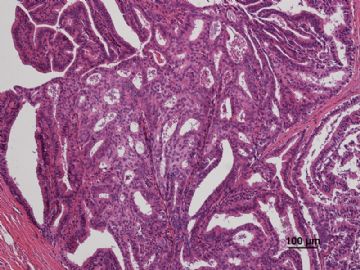
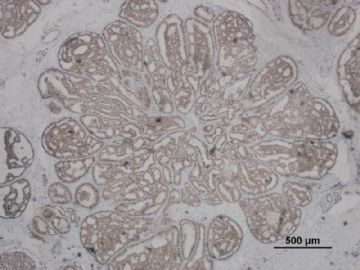





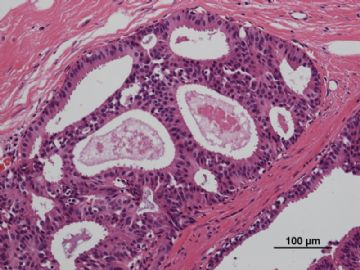

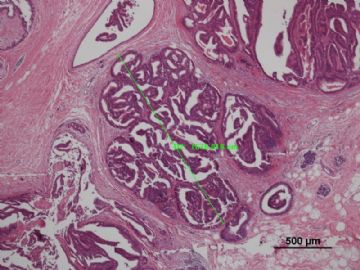
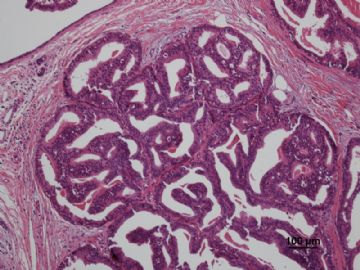
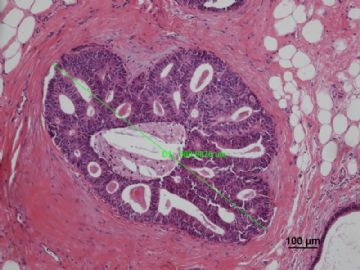






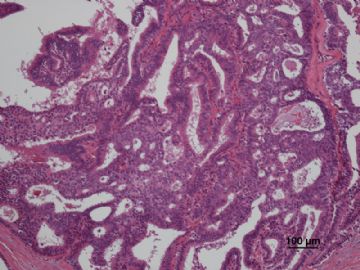


 多谢海上明月老师指导!
多谢海上明月老师指导! 


















