| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
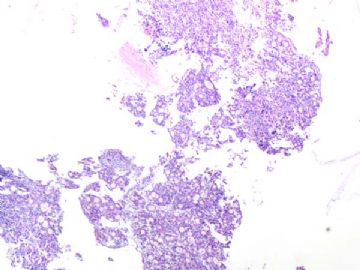
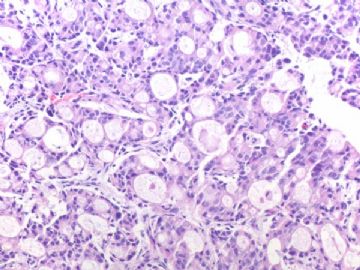
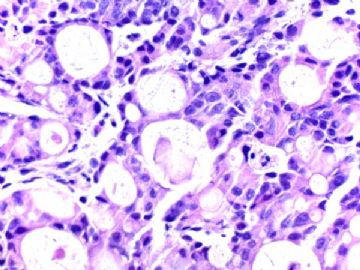
- B291244 y/f vaginal bleeding, endometrial biopsy
(女,44岁,阴道不规则出血,子宫内膜活检)
40x
200x
400x
-
本帖最后由 于 2010-10-02 09:19:00 编辑
相关帖子
本例报道化生性乳腺癌在盆腹腔广泛转移,其中弥漫浸润子宫内膜。
Gynecol Oncol. 2000 Apr;77(1):216-8.
Uterine metastasis from a heterologous metaplastic breast carcinoma simulating a primary uterine malignancy.
Sinkre P, Milchgrub S, Miller DS, Albores-Saavedra J, Hameed A.
Department of Pathology, University of Texas Southwestern Medical Center, Dallas, Texas 75235, USA.
Abstract
OBJECTIVE: To describe the first distant metastasis of a heterologous metaplastic breast carcinoma in the uterus and discuss its differential diagnosis.
METHODS: Light microscopy, immunohistochemistry, and flow cytometry were used to evaluate the tumor.
RESULTS: A 58-year-old woman underwent mastectomy for metaplastic breast carcinoma confined to the breast. She presented 4 years later with vaginal bleeding. The endometrial curettage showed a poorly differentiated carcinoma. She underwent hysterectomy and bilateral salpingo-oophorectomy as well as pelvic and periaortic lymphadenectomy. Clinical and intraoperative findings favored a primary uterine malignancy. The uterus was markedly distorted with multiple gray-white, solid subserosal, and intramural tumor nodules. The tumor diffusely infiltrated the endometrium sparing benign endometrial glands. The tumor nodules were distributed full thickness of the myometrium. These nodules were composed of high-grade malignant epithelial cells with areas of chondroid metaplasia. Extrauterine microscopic tumor was present in left ovary, pelvic, and periaortic lymph nodes. The histologic features and estrogen/progesterone receptors (ER/PR) as well as DNA ploidy analysis of the uterine tumor showed striking similarity with those of the primary metaplastic breast carcinoma. A diagnosis of metastatic metaplastic breast carcinoma in the uterus was rendered.
CONCLUSION: A metastatic heterologous metaplastic breast carcinoma with cartilaginous metaplasia should be considered in the differential diagnosis of heterologous uterine malignant mixed mesodermal tumor (MMMT) and high-grade endometrioid carcinoma with rare foci of cartilage.

- 王军臣
本例报道浸润性小叶癌转移至宫颈。
J Obstet Gynaecol Res. 2007 Aug;33(4):578-80.
Metastasis of lobular breast carcinoma to the cervix.
Perisić D, Jancić S, Kalinović D, Cekerevac M.
Department of Obstetrics and Gynecology, Gornji Milanovac Medical Center, Serbia. perisic@eunet.yu
Abstract
Breast cancers without previous dissemination occasionally result in hematogenous metastases in gynecologic organs, particularly in the cervix. This case report examines a multimodally treated lobular breast cancer patient who was 52 months later diagnosed with malignancy in the uterus. After a tumor was detected at the endocervical and isthmic part of the uterus, a complete hysterectomy with bilateral salpingo-oophorectomy was carried out. The malignant cells were highly positive for carcino-embrional antigen and gross cystic disease fluid protein-15, sensitive breast cancer markers. In conclusion, as breast carcinoma can metastasize to atypical places (cervix, endometrium, etc.), regular surveillance of patients should include gynecologic control

- 王军臣
本例报道浸润性乳腺癌转移至子宫平滑肌瘤中。
J Gynecol Obstet Biol Reprod (Paris). 1993;22(3):243-4.
[Metastasis of breast cancer to a uterine leiomyoma]
[Article in French]
Afriat R, Lenain H, Vuagnat C, Michenet P, Luthier F, Maitre F, Grossetti D.
Service de Gynécologie-Obstétrique, Hôpital Notre-Dame-de-Bon-Secours, Paris.
Abstract
The authors report a case of metastasis of cancer from the breast in a uterine leiomyoma. The metastases into the body of the uterus from extragenital cancers are rare. If they occur they are usually in the myometrium where they are asymptomatic and where the diagnosis is difficult, or in the endometrium where the diagnosis can be made by biopsy after curettage. A few rare cases of metastases in uterine leiomyoma have been reported in the literature. They can be the cause of very sudden increase in size of fibroids with compression of the pelvic organs.

- 王军臣
-
本帖最后由 于 2010-10-03 14:14:00 编辑
下列文献为WT1与乳腺癌的相关文献,供网友们参考。
Pathology. 2010;42(6):534-9.
Armes JE, Bowlay G, Lourie R, Venter DJ, Price G.
Department of Pathology, Mater Health Services, South Brisbane, Queensland, Australia. jane.armes@mater.org.au
Abstract
AIMS: There is a high frequency of serous gynaecological and basal breast cancers in patients with germline BRCA1 mutations. These patients often undergo prophylactic salpingo-oophorectomies, where small tumours may be identified in which morphological distinction between primary serous and metastatic basal breast cancer may be problematic. These two cancer types share similar molecular genetics and have immunophenotypic overlap. We aimed to develop an immunohistochemical panel which could differentiate between these two tumour types.
METHODS: Immunohistochemistry was performed on a training set of 20 serous gynaecological and 20 basal breast cancers, from which a small differential panel was developed. This differential panel was tested in an additional validation set of serous and basal cancers.
RESULTS: WT1, ER and CK5/6 expression successfully differentiated serous gynaecological and basal breast cancers. WT1 and ER expression was significantly more common in serous cancers and CK5/6 expression more common in basal cancers.
CONCLUSION: There is considerable genetic, morphological and immunophenotypic overlap between serous gynaecological and basal breast cancers. A limited panel of WT1, ER and CK5/6 was found to optimally differentiate these tumour types. Differences in the expression of these proteins appear to reflect the expression pattern of their cell of origin, rather than identify aberrant oncogenic pathways.
2.
Indian J Cancer. 2009 Oct-Dec;46(4):303-10.
Gupta S, Joshi K, Wig JD, Arora SK.
Department of Immunopathology, Postgraduate Institute of Medical Education & Research, Chandigarh, India.
Abstract
BACKGROUND: The product of Wilms' tumor suppressor gene (WT1), a nuclear transcription factor, regulates the expression of the insulin-like growth factor (IGF) and transforming growth factor (TGF) systems, both of which are implicated in breast tumorigenesis and are known to facilitate angiogenesis. In the present study, WT1 allelic integrity was examined by Loss of Heterozygosity (LOH) studies in infiltrating breast carcinoma (n=60), ductal carcinoma in situ (DCIS) (n=10) and benign breast disease (n=5) patients, to determine its possible association with tumor progression.
METHODS: LOH at the WT1 locus (11p13) as determined by PCR-RFLP for Hinf1 restriction site and was subsequently examined for its association with intratumoral expression of various growth factors i.e. TGF-beta1, IGF-II, IGF-1R and angiogenesis (VEGF and Intratumoral micro-vessel density) in breast carcinoma.
RESULTS: Six of 22 (27.2%) genetically heterozygous of infiltrating breast carcinoma and 1 of 4 DCIS cases showed loss of one allele at WT1 locus. Histologically, the tumors with LOH at WT1 were Intraductal carcinoma (IDC) and were of grade II and III. There was no correlation in the appearance of LOH at WT1 locus with age, tumor stage, menopausal status, chemotherapy status and lymph node metastasis. The expression of factor IGF-II and its receptor, IGF-1R was significantly higher in carcinoma having LOH at WT1 locus. A positive correlation was observed between the TGF-beta1, VEGF expression and IMD scores in infiltrating carcinoma.
CONCLUSIONS: The current study indicates that the high frequency of loss of allelic integrity at Wilms' tumor suppressor gene-1 locus in high-graded breast tumors is associated with aggressiveness of the tumor.
3.
Cancer Biomark. 2009;5(3):109-16.
Center of Anatomy and Functional Morphology, Mount Sinai School of Medicine, New York, NY, USA.
Abstract
Our previous studies revealed that Wilms' tumor 1 (WT-1) protein was highly expressed in breast myoepithelial (ME) and endothelial cells. As the human breast tissue is rich in ME cells and blood vessels, our current study intended to assess whether WT-1 immunohistochemistry may have dual usages in evaluation of the ME cells and micro-vessel density. Consecutive sections were prepared from breast tumors with co-existing normal, hyperplastic, and neoplastic components. Consecutive sections were immunostained for WT-1 and a panel of ME and endothelial cell markers. From each case, 4-5 randomly selected duct clusters were photographed, and the percentages of positive cells for these molecules were compared. Similar to ME cell marker CD10 and smooth muscle actin (SMA), WT-1 expression was preferentially seen in ME cells, and over 90% of WT-1 positive ME cells were immunoreactive to CD10 and SMA. Distinct WT-1 expression was also seen in endothelial cells, and over 90% of WT-1 positive endothelial cells were positive for blood vessel specific markers. With tumor progression, the percentage and intensity of WT-1 positivity decreased in ME cells, whereas increased in endothelial cells. These finding suggest that WT-1 immunohistochemistry may be used to assess both the ME cells and micro-vessel density.
4.
Int J Biol Sci. 2009;5(1):82-96. Epub 2009 Jan 9.
Xu Z, Wang W, Deng CX, Man YG.
Department of Thyroid and Breast Surgery, China-Japan Union Hospital, Jilin University, Changchun, Jilin, China.
Abstract
Our recent studies revealed that focal alterations in breast myoepithelial cell layers significantly impact the biological presentation of associated epithelial cells. As pregnancy-associated breast cancer (PABC) has a significantly more aggressive clinical course and mortality rate than other forms of breast malignancies, our current study compared tumor suppressor expression in myoepithelial cells of PABC and non-PABC, to determine whether myoepithelial cells of PABC may have aberrant expression of tumor suppressors. Tissue sections from 20 cases of PABC and 20 cases of stage, grade, and age matched non-PABC were subjected to immunohistochemistry, and the expression of tumor suppressor maspin, p63, and Wilms' tumor 1 (WT-1) in calponin positive myoepithelial cells were statistically compared. The expression profiles of maspin, p63, and WT-1 in myoepithelial cells of all ducts encountered were similar between PABC and non-PABC. PABC, however, displayed several unique alterations in terminal duct and lobular units (TDLU), acini, and associated tumor tissues that were not seen in those of non-PABC, which included the absence of p63 and WT-1 expression in a vast majority of the myoepithelial cells, cytoplasmic localization of p63 in the entire epithelial cell population of some lobules, and substantially increasing WT-1 expression in vascular structures of the invasive cancer component. All or nearly all epithelial cells with aberrant p63 and WT-1 expression lacked the expression of estrogen receptor and progesterone receptor, whereas they had a substantially higher proliferation index than their counterparts with p63 and WT-1 expression. Hyperplastic cells with cytoplasmic p63 expression often adjacent to, and share a similar immunohistochemical and cytological profile with, invasive cancer cells. To our best knowledge, our main finings have not been previously reported. Our findings suggest that the functional status of myoepithelial cells may be significantly associated with tumor aggressiveness and invasiveness.
5.
Anticancer Res. 2008 Jul-Aug;28(4B):2155-60.
Department of Surgery, Creighton University Medical School, Omaha, NE 68178, USA.
Abstract
BACKGROUND: The Wilms' tumor suppressor gene, wt1, encodes a zinc-finger protein, WT1, that functions as a transcription regulator. Previous studies have suggested a contradictory role for WT1 in breast cancer development.
MATERIALS AND METHODS: MCF10AT3B cells, a cell line derived from a xenograft model of progressive human proliferative breast disease, were used to study WT1 function in early development of breast cancer. A stable cell line was established from MCF10AT3B cells that ectopically expressed the Wilms' tumor suppressor, WT1. Western blot analysis, in vitro and in vivo growth assays were used to study the effects of constitutive WT1 expression on malignant progression of MCF10AT3B cells.
RESULTS: WT1 expression had a profound effect on several aspects of the cell cycle machinery and inhibited estrogen-stimulated and nonstimulated cell growth in vitro. In nude mice, WT1 expression strongly suppressed estrogen-stimulated tumorigenesis of neoplastigenic MCF10AT3B cells.
CONCLUSION: WT1 plays an important role in maintaining normal growth of mammary epithelial cells and dysregulated WT1 expression may contribute to breast cancer development.
6.
Mod Pathol. 2008 Oct;21(10):1217-23. Epub 2008 May 9.
WT1 immunoreactivity in breast carcinoma: selective expression in pure and mixed mucinous subtypes.
Domfeh AB, Carley AL, Striebel JM, Karabakhtsian RG, Florea AV, McManus K, Beriwal S, Bhargava R.
Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA.
Abstract
Current literature suggests that strong WT1 expression in a carcinoma of unknown origin virtually excludes a breast primary. Our previous pilot study on WT1 expression in breast carcinomas has shown WT1 expression in approximately 10% of carcinomas that show mixed micropapillary and mucinous morphology (Mod Pathol 2007;20(Suppl 2):38A). To definitively assess as to what subtype of breast carcinoma might express WT1 protein, we examined 153 cases of invasive breast carcinomas. These consisted of 63 consecutive carcinomas (contained 1 mucinous tumor), 20 cases with micropapillary morphology (12 pure and 8 mixed), 6 micropapillary 'mimics' (ductal no special type carcinomas with retraction artifacts), 33 pure mucinous carcinomas and 31 mixed mucinous carcinomas (mucinous mixed with other morphologic types). Overall, WT1 expression was identified in 33 carcinomas, that is, 22 of 34 (65%) pure mucinous carcinomas and in 11 of 33 (33%) mixed mucinous carcinomas. The non-mucinous component in these 11 mixed mucinous carcinomas was either a ductal no special type carcinoma (8 cases) or a micropapillary component (3 cases). WT1 expression level was similar in both the mucinous and the non-mucinous components. The degree of WT1 expression was generally weak to moderate (>90% cases) and rarely strong (<10% cases). None of the breast carcinoma subtype unassociated with mucinous component showed WT1 expression.
7.
Hum Pathol. 2008 May;39(5):666-71. Epub 2008 Mar 12.
Moritani S, Ichihara S, Hasegawa M, Endo T, Oiwa M, Yoshikawa K, Sato Y, Aoyama H, Hayashi T, Kushima R.
Department of Pathology and Clinical Laboratories, Nagoya Medical Center, Nagoya, Aichi 460-0001, Japan. moritani@nnh.hosp.go.jp
Abstract
Serous papillary adenocarcinoma of the female genital organs and invasive micropapillary carcinoma of the breast have close histologic similarities. Thus, when these cancers occur synchronously or metachronously in the same patient, it is difficult to determine the primary site. We examined 23 serous papillary adenocarcinomas (16 ovarian, 5 endometrial, and 2 peritoneal) and 37 invasive micropapillary carcinomas of the breast (12 pure and 25 mixed types) on immunohistochemical expression of Wilm's tumor antigen-1 (WT1), CA125, and gross cystic disease fluid protein-15 (GCDFP-15), which have been reported to be useful in the differential diagnosis of primary ovarian carcinomas versus metastatic breast cancer to the ovary. The positive rates of WT1, CA125, and GCDFP-15 in serous papillary adenocarcinomas were 78%, 78%, and 0%, respectively, and the corresponding rates in invasive micropapillary carcinomas were 3%, 40%, and 38%. The CA125-positive rate of invasive micropapillary carcinoma was higher than the rate reported for other types of breast carcinomas. We consider CA125 to be not always useful in the differential diagnosis of serous papillary adenocarcinoma and invasive micropapillary carcinoma. Although the positive rate of WT1 was significantly higher in serous papillary adenocarcinoma than in invasive micropapillary carcinoma, WT1 expression in endometrial serous papillary adenocarcinoma was infrequent (20%). WT1 and GCDFP-15 could be useful markers for the differential diagnosis of ovarian and peritoneal serous papillary adenocarcinoma versus breast invasive micropapillary adenocarcinoma. However, the availability of GCDFP-15 is limited because of the low positive rate of GCDFP-15 in invasive micropapillary carcinomas.

- 王军臣
-
nfykdx2008 离线
- 帖子:734
- 粉蓝豆:28
- 经验:761
- 注册时间:2010-09-08
- 加关注 | 发消息
Mod Pathol. 2008 Oct;21(10):1217-23. Epub 2008 May 9.
WT1 immunoreactivity in breast carcinoma: selective expression in pure and mixed mucinous subtypes.
Domfeh AB, Carley AL, Striebel JM, Karabakhtsian RG, Florea AV, McManus K, Beriwal S, Bhargava R.
Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA.
Abstract
Current literature suggests that strong WT1 expression in a carcinoma of unknown origin virtually excludes a breast primary. Our previous pilot study on WT1 expression in breast carcinomas has shown WT1 expression in approximately 10% of carcinomas that show mixed micropapillary and mucinous morphology (Mod Pathol 2007;20(Suppl 2):38A). To definitively assess as to what subtype of breast carcinoma might express WT1 protein, we examined 153 cases of invasive breast carcinomas. These consisted of 63 consecutive carcinomas (contained 1 mucinous tumor), 20 cases with micropapillary morphology (12 pure and 8 mixed), 6 micropapillary 'mimics' (ductal no special type carcinomas with retraction artifacts), 33 pure mucinous carcinomas and 31 mixed mucinous carcinomas (mucinous mixed with other morphologic types). Overall, WT1 expression was identified in 33 carcinomas, that is, 22 of 34 (65%) pure mucinous carcinomas and in 11 of 33 (33%) mixed mucinous carcinomas. The non-mucinous component in these 11 mixed mucinous carcinomas was either a ductal no special type carcinoma (8 cases) or a micropapillary component (3 cases). WT1 expression level was similar in both the mucinous and the non-mucinous components. The degree of WT1 expression was generally weak to moderate (>90% cases) and rarely strong (<10% cases). None of the breast carcinoma subtype unassociated with mucinous component showed WT1 expression.
The first author above paper was our gyn/breast fellow several years ago. During the study perioid, she did the study and indicated that WT1 can be expressed in about half of mucinous or mucinous related breast ca.
Generally WT1 is negative for breast ca and positive for serous carcinomas especially from ovary. In fact WT1 is positive in almost all sex cord stromal tumors, normal tubual epithelium, normal ovarian stroma et al
-
本帖最后由 于 2010-10-07 21:28:00 编辑
Am J Surg Pathol. 2007 Sep;31(9):1378-86.
Diagnostic utility of WT1 immunostaining in ovarian sertoli cell tumor.
Zhao C, Bratthauer GL, Barner R, Vang R.
Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA. zhaoc@upmc.edu
Author Information
*Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, PA
†Department of Gynecologic and Breast Pathology, Armed Forces Institute of Pathology, Washington, DC
‡Division of Gynecologic Pathology, Johns Hopkins Hospital, Baltimore, MD
Current address: Ross Barner, MD, Department of Pathology, Walter Reed Army Medical Center, Washington, DC.
The opinion and assertions contained herein are the private views of the authors and are not to be construed as official or as representing the views of the Department of the Army or the Department of Defense.
Reprints: Chengquan Zhao, MD, Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, 300 Halket Street, Pittsburgh, PA 15213 (e-mail: zhaoc@upmc.edu).
Abstract
WT1, the Wilms tumor gene product, can be expressed in various tumors from different anatomic sites, including some types of ovarian tumors. Regarding the latter, most studies have focused on surface epithelial-stromal tumors in which serous carcinomas are usually positive and endometrioid carcinomas are negative. Very few studies have specifically investigated this marker in ovarian sex cord-stromal tumors; however, limited data in the literature suggest that WT1 may be frequently expressed in sex cord-stromal tumors. As pure Sertoli cell tumor can be in the histologic differential diagnosis of endometrioid tumors (particularly borderline tumor and carcinoma) and carcinoid, immunostaining for WT1 might be of diagnostic value. Immunohistochemical staining for WT1 was performed in 108 ovarian tumors: pure Sertoli cell tumor (n=26), endometrioid borderline tumor (n=25), classic well-differentiated endometrioid carcinoma (n=23), sertoliform endometrioid carcinoma (n=12), and carcinoid (n=22). Additionally, inhibin and calretinin immunostaining were performed in all cases of Sertoli cell tumor for purposes of comparing expression with WT1. Extent of immunostaining was scored on a 0 to 4+ semiquantitative scale, and immunohistochemical composite scores based on a combination of extent and intensity of immunostaining were calculated in positive cases (possible range, 1 to 12). Nuclear expression of WT1 was present in 96% of Sertoli cell tumors, 16% of endometrioid borderline tumors, 13% of classic well-differentiated endometrioid carcinomas, 25% of sertoliform endometrioid carcinomas, and 0% of carcinoids. In Sertoli cell tumors, expression was diffuse (>50% of positive cells) in all positive cases. When positive in the non-Sertoli cell tumors, the extent of expression tended to be focal to patchy (50% or less positive cells). In Sertoli cell tumors, inhibin and calretinin were expressed in 96% and 54% of cases, respectively. The extent of expression of inhibin tended to be diffuse, similar to WT1; however, the extent of immunostaining for calretinin tended to be focal to patchy. The immunohistochemical composite scores for WT1, inhibin, and calretinin were 11.2, 7.6, and 4.8, respectively. Coordinate patterns for the extent of expression of WT1, inhibin, and calretinin in pure Sertoli cell tumor showed that all 3 markers were positive in 54% of cases; however, 42% were positive for WT1 and inhibin but negative for calretinin. In cases positive for both WT1 and inhibin, expression of both markers was diffuse in 84% of cases, but WT1 was diffuse while inhibin was focal to patchy in 16% of cases. We conclude that ovarian Sertoli cell tumor should be added to the growing list of WT1-positive tumors. This marker is useful for the distinction of Sertoli cell tumor from endometrioid tumors and carcinoid. The diagnostic utility of WT1 in Sertoli cell tumor is similar to inhibin but better than that of calretinin.
Int J Gynecol Pathol. 2008 Oct;27(4):507-14.
SF-1 is a diagnostically useful immunohistochemical marker and comparable to other sex cord-stromal tumor markers for the differential diagnosis of ovarian sertoli cell tumor.
Zhao C, Barner R, Vinh TN, McManus K, Dabbs D, Vang R.
Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania 15213, USA. zhaoc@upmc.edu
Abstract
Immunohistochemistry can be an important part of the diagnosis of Sertoli cell tumor of the ovary, including distinction from non-sex cord-stromal tumors such as the sertoliform variant of endometrioid carcinoma and carcinoid. Several good markers for this differential diagnosis have been identified, particularly inhibin, Wilms tumor 1 gene product (WT1), epithelial membrane antigen, and chromogranin; however, many available markers have limitations to some degree. Steroidogenic factor 1 (SF-1; adrenal 4-binding protein; Ad4BP) is a nuclear transcription factor involved in gonadal and adrenal development. In the testes, SF-1 is expressed in Sertoli cells. Immunohistochemical expression of this marker in ovarian sex cord-stromal tumors, including utility for differential diagnosis, has not been rigorously evaluated. As an extension of our previous immunohistochemical studies of ovarian Sertoli cell tumor, expression of SF-1 and comparison with WT1 and inhibin were assessed in 111 primary ovarian tumors: 27 Sertoli cell tumors, 60 endometrioid tumors (including borderline tumors, conventional well-differentiated carcinomas, and sertoliform variants of carcinoma), and 24 carcinoids. SF-1 was expressed in 100% of Sertoli cell tumors but not in endometrioid tumors or carcinoid. WT1 was expressed in 100% of Sertoli cell tumors and 17% of endometrioid tumors; all carcinoids were negative. Inhibin was expressed in 96% of Sertoli cell tumors and 2% of endometrioid tumors (4% of conventional well-differentiated carcinomas); all carcinoids were negative. The extent of expression of all 3 markers was similar in Sertoli cell tumor but greatest for WT1: 63%, 96%, and 78% of cases showed expression of SF-1, WT1, and inhibin, respectively, in more than 50% of tumor cells. Immunohistochemical composite scores combining both extent and intensity of staining in positive cases were calculated for Sertoli cell tumor (possible range: 1-12). Combined extent/intensity of immunostaining was similar for all 3 markers, but WT1 showed the most robust immunoreactivity in positive cases (mean immunohistochemical composite scores for SF-1, WT1, and inhibin: 6.1, 10.8, and 7.8, respectively). We conclude that for the differential diagnosis with endometrioid tumors and carcinoid of the ovary, SF-1 is a sensitive and specific immunohistochemical marker for Sertoli cell tumor and that SF-1 is diagnostically comparable with other good sex cord-stromal markers.
| 以下是引用cqzhao在2010-10-3 20:09:00的发言:
Above are my two papers related to WT1. Question: Is WT1 useful to distunguish breast ca from endometrial ca? Is WT1 useful to distinguish breast ca from endometrioid ca? |
译:
以上是我的两篇论文,与WT-1有关。
问题:
1 WT1有助于区分乳腺癌与内膜癌吗?
2 WT1有助于区分乳腺癌与内膜样癌吗?

华夏病理/粉蓝医疗
为基层医院病理科提供全面解决方案,
努力让人人享有便捷准确可靠的病理诊断服务。
WT-1 可以鉴别乳腺微乳头状癌和乳腺转移性卵巢浆液性乳头状癌。此病例HE形态不符合一般(conventional) 内膜样腺癌,有可能是子宫内膜浆液性癌等。
查阅文献后发现子宫内膜浆液性癌WT-1的表达率(10%)明显低于卵巢的浆液性癌。显然,WT-1 对此病例的鉴别诊断意义不大。
在Dr. Zhao的研究中内膜样腺癌WT-1的表达率为13%。
子宫内膜样腺癌中特殊类型中还包括伴有支持细胞样结构(endometrioid adenocarcinoma with sertoliform pattern)的变型, 但是此病例形态学不符合。

- xljin8
-
本帖最后由 于 2010-10-07 21:32:00 编辑
Agree with Dr. Jin. WT1 can be useful for distinguishing breast ca from ovarian serous ca. Ovarian serous ca is the most common ovarian ca which are positive for WT1 in 85-90% of cases. Most breast carcinomas are negative for WT1. Most endometrioid carcinomas are negative for WT1. A ew percentage of cases are positive in my study, but all of them are weakly and focally positive.
Conclusion: WT1 is useless marker to differ the breast ca from endometrioid ca in most situation.
(同意Dr.Jin
WT1有助于区分乳腺癌和卵巢浆液性癌。卵巢浆液性癌是最常见的卵巢癌,WT1阳性率85-90%。大多数乳腺癌WT1阴性。大多数内膜样癌WT1阴性。我的研究中少数病例阳性,但仅为局灶、弱阳性。
结论:WT1大多数情况下不能区分乳腺癌和内膜样癌。)