| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
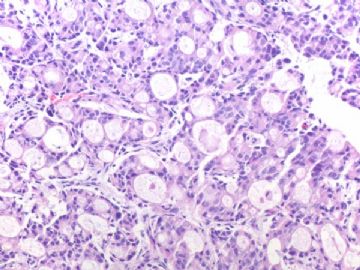
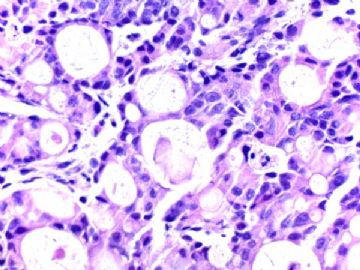
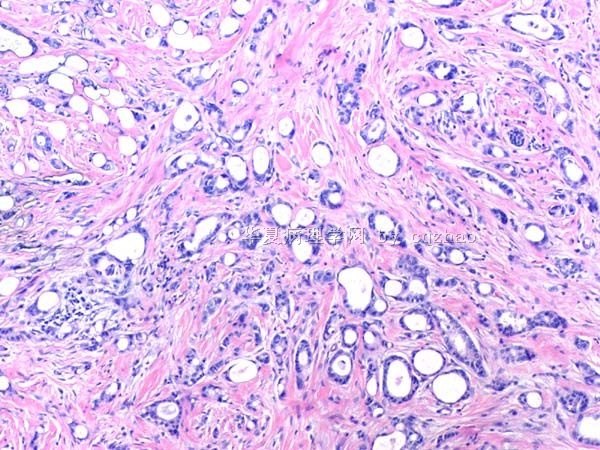
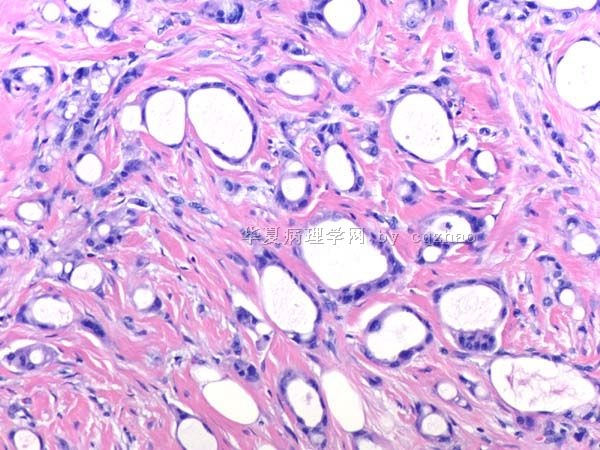
- B291244 y/f vaginal bleeding, endometrial biopsy
(女,44岁,阴道不规则出血,子宫内膜活检)
40x
200x
400x
-
本帖最后由 于 2010-10-02 09:19:00 编辑
相关帖子
| 以下是引用qianxun在2010-11-10 7:52:00的发言:
Dr. Zhao: I am not sure it is breast primary based on ER positive. Did you do DNA mapping for these two tumors? Thanks. Qianxun |
Thank your comment. We made the dx based on clinical hx, cytomorphology and stains. I am not 100% sure the breast primary
This is why my dx is Adenocarcinoma (see comment)
Comment: described the findings of breast and em bx specimens. Favor breast primary based on cytomophologic and IHC features
I did not do DNA mapping. Often I feel it is not very useful.
-
本帖最后由 于 2010-10-27 22:04:00 编辑
Agree above evaluation.
Both breast and current em specimens show weak ER positive.
I signed out the case:
Adenocarcinoma.
Comment: described the findings of breast and em bx specimens. Favor breast primary based on cytomophologic and IHC features.
译:
同意上述分析。
乳腺标本和现在的内膜标本均呈ER弱阳性。
我签发的报告:
腺癌。
注:描述乳腺和内膜标本的形态。根据细胞形态和免疫组化特征倾向乳腺原发。
-
本帖最后由 于 2010-10-07 21:34:00 编辑
The glands in the endometrial biopsy specimenare weakly positive for ER, focally and weakly positive for mammaglobin, and negative for GCDFP.
1. What is your thought now?
2. Is it useful for mammaglobin stain in this case?
3. What percentage of breast carcinomas is positive for GCDFP?
(内膜活检标本中,腺体呈ER弱阳性,mammaglobin局灶弱阳性,GCDFP15阴性。
1、现在考虑什么?
2、乳腺癌的GCDFP15阳性率是多少?)
-
本帖最后由 于 2010-10-07 21:32:00 编辑
Agree with Dr. Jin. WT1 can be useful for distinguishing breast ca from ovarian serous ca. Ovarian serous ca is the most common ovarian ca which are positive for WT1 in 85-90% of cases. Most breast carcinomas are negative for WT1. Most endometrioid carcinomas are negative for WT1. A ew percentage of cases are positive in my study, but all of them are weakly and focally positive.
Conclusion: WT1 is useless marker to differ the breast ca from endometrioid ca in most situation.
(同意Dr.Jin
WT1有助于区分乳腺癌和卵巢浆液性癌。卵巢浆液性癌是最常见的卵巢癌,WT1阳性率85-90%。大多数乳腺癌WT1阴性。大多数内膜样癌WT1阴性。我的研究中少数病例阳性,但仅为局灶、弱阳性。
结论:WT1大多数情况下不能区分乳腺癌和内膜样癌。)
Int J Gynecol Pathol. 2008 Oct;27(4):507-14.
SF-1 is a diagnostically useful immunohistochemical marker and comparable to other sex cord-stromal tumor markers for the differential diagnosis of ovarian sertoli cell tumor.
Zhao C, Barner R, Vinh TN, McManus K, Dabbs D, Vang R.
Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania 15213, USA. zhaoc@upmc.edu
Abstract
Immunohistochemistry can be an important part of the diagnosis of Sertoli cell tumor of the ovary, including distinction from non-sex cord-stromal tumors such as the sertoliform variant of endometrioid carcinoma and carcinoid. Several good markers for this differential diagnosis have been identified, particularly inhibin, Wilms tumor 1 gene product (WT1), epithelial membrane antigen, and chromogranin; however, many available markers have limitations to some degree. Steroidogenic factor 1 (SF-1; adrenal 4-binding protein; Ad4BP) is a nuclear transcription factor involved in gonadal and adrenal development. In the testes, SF-1 is expressed in Sertoli cells. Immunohistochemical expression of this marker in ovarian sex cord-stromal tumors, including utility for differential diagnosis, has not been rigorously evaluated. As an extension of our previous immunohistochemical studies of ovarian Sertoli cell tumor, expression of SF-1 and comparison with WT1 and inhibin were assessed in 111 primary ovarian tumors: 27 Sertoli cell tumors, 60 endometrioid tumors (including borderline tumors, conventional well-differentiated carcinomas, and sertoliform variants of carcinoma), and 24 carcinoids. SF-1 was expressed in 100% of Sertoli cell tumors but not in endometrioid tumors or carcinoid. WT1 was expressed in 100% of Sertoli cell tumors and 17% of endometrioid tumors; all carcinoids were negative. Inhibin was expressed in 96% of Sertoli cell tumors and 2% of endometrioid tumors (4% of conventional well-differentiated carcinomas); all carcinoids were negative. The extent of expression of all 3 markers was similar in Sertoli cell tumor but greatest for WT1: 63%, 96%, and 78% of cases showed expression of SF-1, WT1, and inhibin, respectively, in more than 50% of tumor cells. Immunohistochemical composite scores combining both extent and intensity of staining in positive cases were calculated for Sertoli cell tumor (possible range: 1-12). Combined extent/intensity of immunostaining was similar for all 3 markers, but WT1 showed the most robust immunoreactivity in positive cases (mean immunohistochemical composite scores for SF-1, WT1, and inhibin: 6.1, 10.8, and 7.8, respectively). We conclude that for the differential diagnosis with endometrioid tumors and carcinoid of the ovary, SF-1 is a sensitive and specific immunohistochemical marker for Sertoli cell tumor and that SF-1 is diagnostically comparable with other good sex cord-stromal markers.
-
本帖最后由 于 2010-10-07 21:28:00 编辑
Am J Surg Pathol. 2007 Sep;31(9):1378-86.
Diagnostic utility of WT1 immunostaining in ovarian sertoli cell tumor.
Zhao C, Bratthauer GL, Barner R, Vang R.
Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA. zhaoc@upmc.edu
Author Information
*Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, PA
†Department of Gynecologic and Breast Pathology, Armed Forces Institute of Pathology, Washington, DC
‡Division of Gynecologic Pathology, Johns Hopkins Hospital, Baltimore, MD
Current address: Ross Barner, MD, Department of Pathology, Walter Reed Army Medical Center, Washington, DC.
The opinion and assertions contained herein are the private views of the authors and are not to be construed as official or as representing the views of the Department of the Army or the Department of Defense.
Reprints: Chengquan Zhao, MD, Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, 300 Halket Street, Pittsburgh, PA 15213 (e-mail: zhaoc@upmc.edu).
Abstract
WT1, the Wilms tumor gene product, can be expressed in various tumors from different anatomic sites, including some types of ovarian tumors. Regarding the latter, most studies have focused on surface epithelial-stromal tumors in which serous carcinomas are usually positive and endometrioid carcinomas are negative. Very few studies have specifically investigated this marker in ovarian sex cord-stromal tumors; however, limited data in the literature suggest that WT1 may be frequently expressed in sex cord-stromal tumors. As pure Sertoli cell tumor can be in the histologic differential diagnosis of endometrioid tumors (particularly borderline tumor and carcinoma) and carcinoid, immunostaining for WT1 might be of diagnostic value. Immunohistochemical staining for WT1 was performed in 108 ovarian tumors: pure Sertoli cell tumor (n=26), endometrioid borderline tumor (n=25), classic well-differentiated endometrioid carcinoma (n=23), sertoliform endometrioid carcinoma (n=12), and carcinoid (n=22). Additionally, inhibin and calretinin immunostaining were performed in all cases of Sertoli cell tumor for purposes of comparing expression with WT1. Extent of immunostaining was scored on a 0 to 4+ semiquantitative scale, and immunohistochemical composite scores based on a combination of extent and intensity of immunostaining were calculated in positive cases (possible range, 1 to 12). Nuclear expression of WT1 was present in 96% of Sertoli cell tumors, 16% of endometrioid borderline tumors, 13% of classic well-differentiated endometrioid carcinomas, 25% of sertoliform endometrioid carcinomas, and 0% of carcinoids. In Sertoli cell tumors, expression was diffuse (>50% of positive cells) in all positive cases. When positive in the non-Sertoli cell tumors, the extent of expression tended to be focal to patchy (50% or less positive cells). In Sertoli cell tumors, inhibin and calretinin were expressed in 96% and 54% of cases, respectively. The extent of expression of inhibin tended to be diffuse, similar to WT1; however, the extent of immunostaining for calretinin tended to be focal to patchy. The immunohistochemical composite scores for WT1, inhibin, and calretinin were 11.2, 7.6, and 4.8, respectively. Coordinate patterns for the extent of expression of WT1, inhibin, and calretinin in pure Sertoli cell tumor showed that all 3 markers were positive in 54% of cases; however, 42% were positive for WT1 and inhibin but negative for calretinin. In cases positive for both WT1 and inhibin, expression of both markers was diffuse in 84% of cases, but WT1 was diffuse while inhibin was focal to patchy in 16% of cases. We conclude that ovarian Sertoli cell tumor should be added to the growing list of WT1-positive tumors. This marker is useful for the distinction of Sertoli cell tumor from endometrioid tumors and carcinoid. The diagnostic utility of WT1 in Sertoli cell tumor is similar to inhibin but better than that of calretinin.
The first author above paper was our gyn/breast fellow several years ago. During the study perioid, she did the study and indicated that WT1 can be expressed in about half of mucinous or mucinous related breast ca.
Generally WT1 is negative for breast ca and positive for serous carcinomas especially from ovary. In fact WT1 is positive in almost all sex cord stromal tumors, normal tubual epithelium, normal ovarian stroma et al
Mod Pathol. 2008 Oct;21(10):1217-23. Epub 2008 May 9.
WT1 immunoreactivity in breast carcinoma: selective expression in pure and mixed mucinous subtypes.
Domfeh AB, Carley AL, Striebel JM, Karabakhtsian RG, Florea AV, McManus K, Beriwal S, Bhargava R.
Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA.
Abstract
Current literature suggests that strong WT1 expression in a carcinoma of unknown origin virtually excludes a breast primary. Our previous pilot study on WT1 expression in breast carcinomas has shown WT1 expression in approximately 10% of carcinomas that show mixed micropapillary and mucinous morphology (Mod Pathol 2007;20(Suppl 2):38A). To definitively assess as to what subtype of breast carcinoma might express WT1 protein, we examined 153 cases of invasive breast carcinomas. These consisted of 63 consecutive carcinomas (contained 1 mucinous tumor), 20 cases with micropapillary morphology (12 pure and 8 mixed), 6 micropapillary 'mimics' (ductal no special type carcinomas with retraction artifacts), 33 pure mucinous carcinomas and 31 mixed mucinous carcinomas (mucinous mixed with other morphologic types). Overall, WT1 expression was identified in 33 carcinomas, that is, 22 of 34 (65%) pure mucinous carcinomas and in 11 of 33 (33%) mixed mucinous carcinomas. The non-mucinous component in these 11 mixed mucinous carcinomas was either a ductal no special type carcinoma (8 cases) or a micropapillary component (3 cases). WT1 expression level was similar in both the mucinous and the non-mucinous components. The degree of WT1 expression was generally weak to moderate (>90% cases) and rarely strong (<10% cases). None of the breast carcinoma subtype unassociated with mucinous component showed WT1 expression.