| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
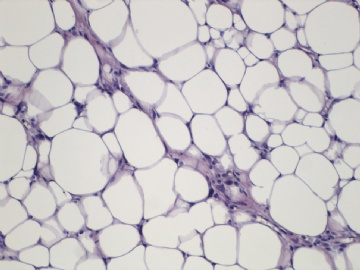
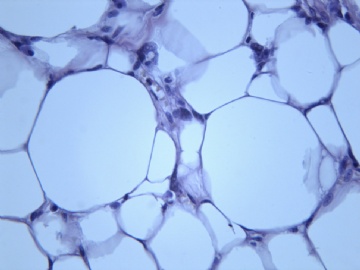
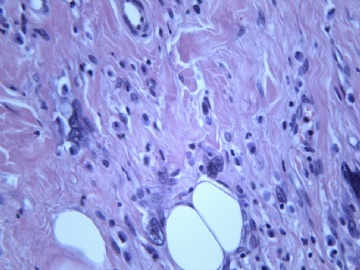
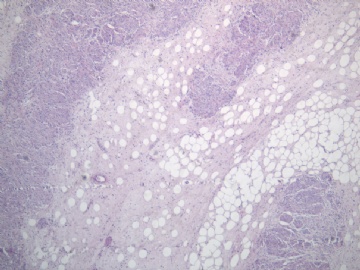
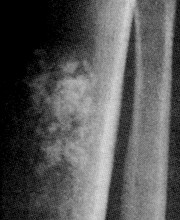
- B2238纵隔多结节性肿物,期待您的高见!
-
本帖最后由 于 2010-08-31 19:02:00 编辑
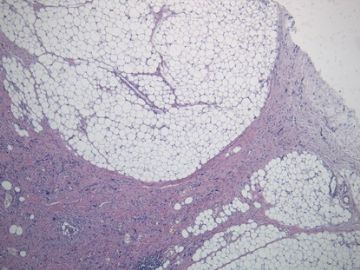
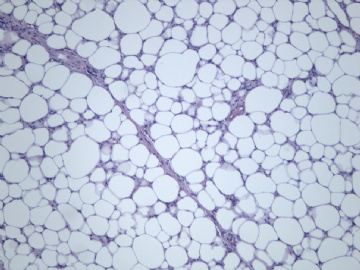
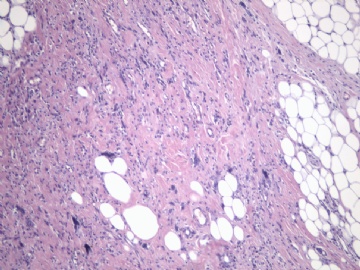
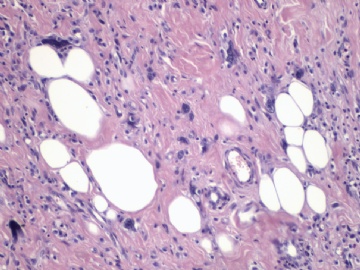
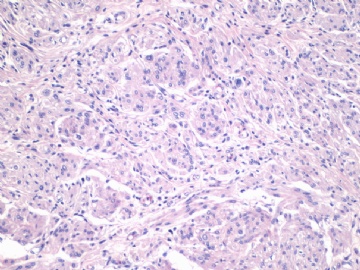
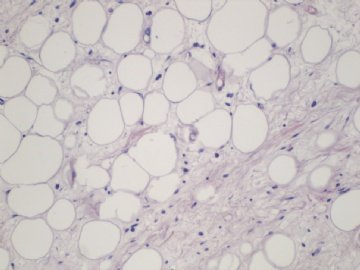
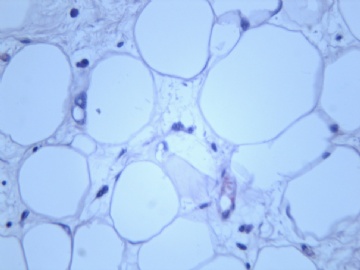
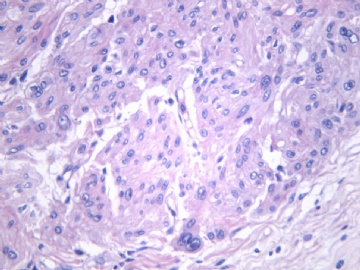
非常好的病例,根据形态学和IHC标记结果。前7张图为高分化脂肪肉瘤,后5张图是脂肪+平滑肌肿瘤, 二者应该以一元论解释。
如何诊断?
1)脂肪肉瘤,去分化型,低度恶性
2)去分化脂肪肉瘤(去分化成分平滑肌肉瘤)?
3)高分化脂肪肉瘤伴有低级别去分化(Well differentiated liposarcoma with low-grade dedifferentiation)?
4)高分化脂肪肉瘤伴平滑肌分化(脂肪平滑肌肉瘤 Lipoleiomyosarcoma)?
5)高分化脂肪肉瘤伴有微小去分化(高度恶性肉瘤,面积<1cm, Well differentiated liposarcoma with minimal dedifferentiation)
谢谢!

- xljin8
- 文章:1662
- 积分:9280
- 经验:2030
- 注册:2009-3-4 3:39
|
| ||||||||
非常赞赏金主任的精辟分析。
平滑肌组织有异型,而且是在脂肪肉瘤内的灶性区域,可能还是按一元论伴异源性分化报告比较好。那就选择金主任上述的4)高分化脂肪肉瘤伴局灶平滑肌分化(局灶含有高分化平滑肌肉瘤成分)。IHC标记(S-100、SMA、desmin、caldesmon、Ki-67)鉴别。

- 王军臣
|
非常好的病例,根据形态学和IHC标记结果。前7张图为高分化脂肪肉瘤,后5张图是脂肪+平滑肌肿瘤, 二者应该以一元论解释。 如何诊断? 1)脂肪肉瘤,去分化型,低度恶性 2)去分化脂肪肉瘤(去分化成分平滑肌肉瘤)? 3)高分化脂肪肉瘤伴有低级别去分化(Well differentiated liposarcoma with low-grade dedifferentiation)? 4)高分化脂肪肉瘤伴平滑肌分化(脂肪平滑肌肉瘤 Lipoleiomyosarcoma)? 5)高分化脂肪肉瘤伴有微小去分化(高度恶性肉瘤,面积<1cm, Well differentiated liposarcoma with minimal dedifferentiation) |
-
本帖最后由 于 2010-12-22 21:21:00 编辑
| 以下是引用sanxia在2010-9-1 12:28:00的发言: 金主任,如果是您的病例,能说说您如何诊断吗?请多赐教!谢谢! |
赐教不敢当,还是相互学习。
我想有几点要了解的:
1)脂肪性肿瘤中伴有非脂肪细胞成分是非常或最常见的, 如有血管、梭形纤维细胞、平滑肌、软骨、骨细胞。为什么?实际上脂肪组织中有许多的“干细胞”。比如在大网膜中提取干细胞技术就是运用此特点。因此,在软组织肿瘤中出现“其他间叶成分”最常见是脂肪性肿瘤。
2)不典型脂肪肿瘤/高分化脂肪肉瘤是软组织最常见的肉瘤,肿瘤的不成熟状态更可能发生“异分化”。
3)“去分化”概念是Dahlin 最先用于高分化软骨肉瘤,在后期组织学转变为高级别肉瘤。但是这种现象在软组织肿瘤中很常见,并被用于其它低级别的软组织肉瘤,比如皮质旁骨肉瘤、脊索瘤和高分化脂肪肉瘤。由于去分化后,最常见的形态学改变是MFH,因此有学者提出“MFH是软组织肉瘤最后的共同之路”
4)传统上“去分化脂肪肉瘤”定义为高分化脂肪肉瘤中出现非脂肪源性高度恶性肉瘤,如纤维肉瘤、MFH等。因此诊断“去分化”必须符合2点,1)非脂肪源性,2)高级别肉瘤。
5)高分化脂肪肉瘤是否允许出现实体性高分化的梭形细胞和平滑肌分化区域?这是本病例与去分化脂肪肉瘤鉴别诊断的关键。
6)非常同意Dr.海上明月的上述观点。如果平滑肌成分也是低级别,就不符合诊断“去分化脂肪肉瘤的标准”
7)还要注意,研究发现去分化脂肪肉瘤中,区分化成分少(<10%), 对预后影响不大。因此有人提出用局灶去分化的名称。
8)对于诊断“去分化”,又后加“低度恶性”, 临床医生很难理解,也许会提问“此诊断对治疗和预后有什么临床意义?”
谢谢您的少见病例!

- xljin8
-
jiajing1115 离线
- 帖子:41
- 粉蓝豆:21
- 经验:53
- 注册时间:2008-12-22
- 加关注 | 发消息
-
本帖最后由 于 2010-09-04 04:02:00 编辑
向各位老师学习了!
请问金主任:3)高分化脂肪肉瘤伴有低级别去分化(Well differentiated liposarcoma with low-grade dedifferentiation)?具体的低级别去分化都包括哪些
2)去分化脂肪肉瘤(去分化成分平滑肌肉瘤)?平滑肌肉瘤部分占瘤体的多少才报去分化?海上明月老师说的:高分化脂肪肉瘤伴局灶平滑肌分化(局灶含有高分化平滑肌肉瘤成分)。包括了平滑肌肉瘤成分,为什么不报去分化呢?
见笑了,谢谢各位老师
-
本帖最后由 于 2010-12-22 21:26:00 编辑
| 以下是引用sanxia在2010-9-3 18:46:00的发言: 谢谢金主任,学习了! 不过,WHO和ACKERMAN外科病理学上都说“去分化成分可以是低级别的”,不知该如何理解?谢谢! |
非常好的问题! 试做解答:
1) 对于脂肪源性恶性肿瘤研究发展比较快的阶段是在1990年后。到2000左右基本分类已大致确定。(1)不典型脂肪肿瘤(主要位于浅表)/高分化脂肪肉瘤(体腔)。高分化脂肪肉瘤又细分为:脂肪瘤样脂肪肉瘤、炎症型脂肪肉瘤、硬化型脂肪肉瘤、去分化脂肪肉瘤。(2)黏液脂肪肉瘤/圆形细胞脂肪肉瘤。(3)多形性脂肪肉瘤。
2) ‘不典型’脂肪肿瘤的概念引入是为了避免对形态学为高分化脂肪肉瘤,但肿瘤发生在躯干和四肢浅表部位,往往临床发现早,手术易于完整切除、复发率低的这些病例治疗过度。就像把发生在皮下浅表组织的MFH称为“不典型黄色纤维瘤”一样。
3) 最初Dahlin提出的“去分化”的概念是指低度恶性的皮质旁骨肉瘤转变为高度恶性的骨肉瘤或纤维肉瘤/MFH, 原本无“异源性”要求。1979 Evan提出去分化脂肪肉瘤的概念, 并提出“非脂肪源性”和“高级别”二项要求。
4) 交界性或低度恶性肿瘤“去分化”后,一般生物学行为转变为高度恶性。但是,其恶性程度还要取决于“去分化区域“的大小和恶性度。
5) 文献中低度恶性去分化脂肪肉瘤的文章是强调“另一种肉瘤成分”的恶性程度低,区别于低度恶性脂肪肉瘤去分化后出现高度恶性转变。
6) 文献中低级别非脂肪性肉瘤成分主要为2种类型;a.平滑肌肉瘤[1];b.骨肉瘤[2]。
7) 在高分化脂肪肉瘤中出现梭形细胞[3]、平滑肌、软骨、骨组织时, 要根据其形态学特征来确定其良恶性,恶性程度为为低级别还是高级别。个人认为如果高分化脂肪肉瘤中出现低级别异源性肉瘤成分,如高分化脂肪肉瘤伴有部分高分化平滑肌肉瘤成分,高分化骨肉瘤,不提倡诊断为去分化脂肪肉瘤。真如文献1-3中指出的那样。
8)如果是原发肿瘤出现高分化脂肪肉瘤和另外一种高分化肉瘤,也可认为是‘恶性间叶瘤’。当然,有少数去分化脂肪肉瘤在首次手术标本就有高分化脂肪肉瘤和界限明显的高度恶性肉瘤区域。如果异源性成分是出现在复发性高分化脂肪肉瘤中,它可能提示肿瘤分化基因不稳定的显性化或出现了继发性分子事件。
以上纯属个人观点,仅供参考,错误之处请指正。 谢谢!
1) Folpe AL, Weiss SW.
Lipoleiomyosarcoma (well-differentiated liposarcoma with leiomyosarcomatous differentiation): a clinicopathologic study of nine cases including one with dedifferentiation. Am J Surg Pathol. 2002;26:742-9.
Leiomyosarcomatous (LMS) differentiation is a rare event in liposarcoma (LPS) and may consist of either well-differentiated liposarcoma (WDL) with an intrinsic smooth muscle component, so-called "lipoleiomyosarcoma," (L-LMS) or dedifferentiated liposarcoma having smooth muscle differentiation in the dedifferentiated zones. The latter are high-grade sarcomas, whereas the behavior of the former group is uncertain. Specifically, it is not clear whether the presence of LMS negatively affects the prognosis. We present our experience with
nine cases, the largest to date. The patients (seven male, two female) ranged in age from 42 to 65 years (mean 54 years). The tumors were usually large (2 to >40 cm [mean 17 cm]) tumors in the retroperitoneum (two cases), paratesticular-inguinal region (three cases), mediastinum (one case), lung (one case), abdomen (one case), and popliteal fossa (one case). The nine cases qualified as L-LMS and showed typical WDL with a multifocal, gradual transition into smooth muscle areas. The latter areas accounted for a variable portion of the lesions (range 5-90%) and were of low cellularity, mild to moderate nuclear atypia, and low mitotic activity. These areas seemed to arise from or blend with the smooth muscle in the walls of large vessels within the tumor. One case showed areas of dedifferentiation consisting of actin and desmin-negative, high-grade sarcoma. Follow-up in seven cases (range 26-312 months; mean 119 months) showed multiple local recurrences in seven patients and no metastases. Three patients are currently without evidence of disease (follow-up duration 26-312 months; mean 144 months) and four patients are alive with progressive disease (follow-up duration 60-132 months; mean 99 months). Our study suggests that L-LMS is a dual lineage sarcoma as evidenced by the fact that the smooth muscle component is often multifocal, not necessarily found in close association with the atypical changes in fat, and seemingly originates from atypical ("in situ") changes in the vessel wall. The LMS component, which is typically low grade, does not adversely affect the overall behavior of the tumor, which is similar to that of conventional WDL. LMS in L-LMS should not be misconstrued as evidence of low-grade dedifferentiation, a phenomenon that identifies a more unstable and potentially metastasizing lesion.
2. Yoshida A, Ushiku T, Motoi T, Shibata T, Fukayama M, Tsuda H.
Well-differentiated liposarcoma with low-grade osteosarcomatous component: an underrecognized variant.
Am J Surg Pathol. 2010;34:1361-6.
Mature bone formation in well-differentiated liposarcoma and dedifferentiated liposarcoma has been described as a reactive or "metaplastic" change in most reports, and its neoplastic nature has not been widely appreciated. We herein describe 9 cases of well-differentiated/dedifferentiated liposarcoma with distinct areas of fibroosseous tissue histologically indistinguishable from low-grade osteosarcomas, that is, parosteal osteosarcoma or low-grade central osteosarcoma. The tumors affected middle-aged to elderly patients, and occurred in the retroperitoneum and deep soft tissue of the extremities without connection to the skeletal system. Grossly, all the tumors showed biphasic appearance with lipogenic and osteogenic area, the latter representing 5% to 50% of the total tumor volume. Histologically, the lipogenic component exhibited typical histology of well-differentiated liposarcoma, whereas the osteogenic area consisted of fibroosseous tissue with numerous mature neoplastic bone trabeculae largely lacking osteoblastic rimming, with intervening fascicles of spindle cell proliferation showing low nuclear grade. All samples were positive for MDM2 and/or CDK4 on immunohistochemical analysis; the antibodies stained many osteocytes, indicating that the bone is neoplastic rather than reactive. Three cases showed high-grade osteosarcomatous transformation juxtaposed to the low-grade osteosarcomatous component, reminiscent of the "dedifferentiation" phenomenon of skeletal low-grade osteosarcoma. Follow-up revealed local recurrence in 4 cases, but no distant metastases were documented. Recognition of this earlier underappreciated subtype of well differentiated/dedifferentiated liposarcoma is important, because the fibroosseous component may seem so bland that it may be confused with benign metaplasia such as myositis ossificans, or conversely, the lipomatous component may be inconspicuous that it may be dismissed as normal fat, and such misinterpretation may potentially result in suboptimal treatment.
3) Dei Tos AP, Mentzel T, Newman PL, Fletcher CD.
Spindle cell liposarcoma, a hitherto unrecognized variant of liposarcoma. Analysis of six cases
Am J Surg Pathol. 1994;18:913-21.
A series of six cases of a previously unrecognized variant of liposarcoma characterized by a prominent spindle cell component is reported herein. Clinically, all of the tumors arose in adults and developed around the shoulder girdle or upper limbs; all but one arose in subcutaneous tissue. Three patients developed multiple local recurrences over a period of 4-20 years. Recurrences in one case were purely lipoma-like. Following dedifferentiation in a recurrence,one patient developed distant metastases and eventually died, 46 months after the primary excision. Grossly, these lesions are characterized by multinodularity, and microscopically they show a relatively bland spindle cell proliferation arranged in fascicles and whorls and set in a variably myxoid stroma. The spindle cell areas are accompanied by an adipocytic component, which exhibits the morphologic features required for inclusion in the well-differentiated liposarcoma-atypical lipoma group, including definite lipoblasts. Main differential diagnoses include benign lesions such as spindle cell lipoma and diffuse neurofibroma, as well as dermatofibrosarcoma protuberans and other malignancies such as sclerosing liposarcoma, low-grade myxofibrosarcoma,low-grade malignant peripheral nerve sheath tumor, and low-grade fibromyxoid sarcoma. In view of their distinctive histologic appearance, and because aggressive clinical behavior was observed despite their superficial location, we propose that these lesions be regarded as spindle cell variants of well-differentiated liposarcoma.

- xljin8