| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- 20100727- 前列腺穿刺够癌吗?
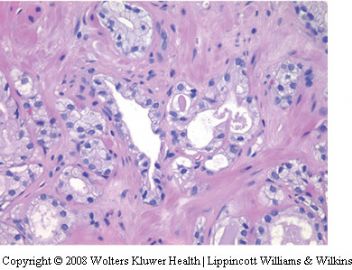
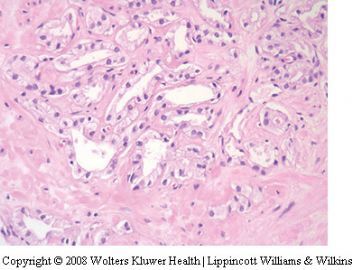
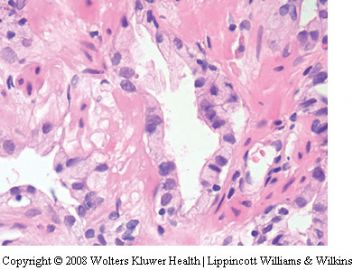
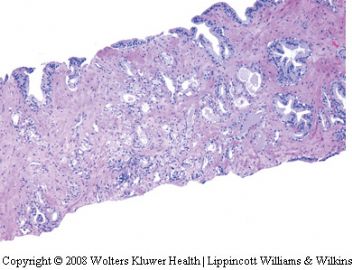
泡沫腺腺癌是前列腺腺泡性腺癌的变异型之一。活检时很少遇见。它的形态特点比较特别。主要表现在癌细胞呈丰富的泡沫样空亮的胞浆,其核浆比很低。诊断时需注意如下几点:
(一)细胞核小是造成泡沫腺腺癌诊断困难的主要因素:核相对于经典腺泡性腺癌的胞核要小得多,也不像经典型腺泡性腺癌那样核仁明显。可以说几乎见不到核仁。在前列腺活检病理诊断中,难就难在见不到胞核的异型性以致不敢诊断癌,看起来更像良性前列腺分泌性上皮的细胞核,甚至还要小。然而,泡沫腺腺癌的细胞核是深染的,多少有些大小不是很一致。
(二)腺体结构及细胞形态:由泡沫样空亮胞浆的细胞构成,胞浆丰富,核浆比较小。细胞呈矮立方或柱状(核较靠近基底),多呈单层排列,由于胞浆泡沫样且透亮或极其淡染,故显示出细胞界限清晰(细胞膜所在位置呈线状界限)。腺腔内可含红染的分泌物。没有基底细胞。但腺体构筑或浸润与经典腺泡性腺癌一致。
(三)泡沫腺腺癌不是低级别腺癌,绝大多数判为中等级别,千万不要因为泡沫腺的良性结构被误认为是低级别腺癌。
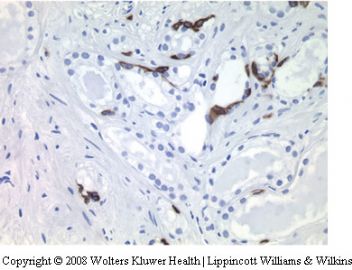
(四)标记物标记与经典性腺泡性腺癌一样。
学习随记,仅供参考。

- 王军臣
| 以下是引用doudou20080626在2010-8-23 17:30:00的发言: 学习了,前段时间遇到一例,本人考虑为高分化癌,科主任直接给我否定了。其实它的PSA也是25,P63部分腺体不表达。迷惑的是我们的P504S阴性。 |
P504S这个抗体做出来有时是会使人困惑,这其中有技术方面的原因,也有抗体供应方面的问题。
技术上的问题主要可能有固定是否及时的因素,因为P504s是一种参与脂肪酸支链及其之间产物代谢的酶,如果固定不及时或固定不良,容易导致该酶降解。技术上的因素还有抗原修复的步骤没掌握好火候,这个平常要拿阳性对照片去试,看什么样的修复温度和修复时间比较合适。还有一些IHC操作细节问题。
抗体供应上的问题主要是这个抗体使用的相对较少,所以在购买前供应商那里可能储藏的时间较长,其效价降低,特别是即用型抗体,买来后很少用而久存,也会降低效价。这时,解决的办法就是在即用型抗体中,加几滴未稀释的原始一抗,以提供有效抗体效价,说不定使用起来效果会好些。
仅供参考。

- 王军臣
-
lantian0508 离线
- 帖子:1250
- 粉蓝豆:42
- 经验:1495
- 注册时间:2007-08-01
- 加关注 | 发消息
-
本帖最后由 于 2010-08-01 01:44:00 编辑
Benign mimickers of prostatic adenocarcinoma
John R Srigley
Department of Pathology and Molecular Medicine, McMaster University, Hamilton, ON, Canada
The diagnosis of prostatic adenocarcinoma, especially when present in small amounts, is often challenging.Before making a diagnosis of carcinoma, it is prudent for the pathologist to consider the various benign patterns and processes that can simulate prostatic adenocarcinoma. A useful method of classifying benign mimickers is in relationship to the major growth patterns depicted in the classical Gleason diagram. The four major patterns are small gland, large gland, fused gland and solid. Most mimickers fit within the small gland category and the most common ones giving rise to false-positive cancer diagnosis are atrophy, post-atrophic hyperplasia, atypical adenomatous hyperplasia and seminal vesicle-type tissue. A number of other
histoanatomic structures such as Cowper’s gland, verumontanum mucosal glands, mesonephric glands and paraganglionic tissue may be confused with adenocarcinoma. Additionally, metaplastic and hyperplastic processes within the prostate may be confused with adenocarcinoma. Furthermore, inflammatory processes including granulomatous prostatitis, xanthogranulomatous prostatitis and malakoplakia may simulate highgrade adenocarcinoma. Atypical adenomatous hyperplasia (adenosis), a putative precursor of transition zone adenocarcinoma, has overlapping features with low-grade adenocarcinoma and may cause problems in differential diagnosis, especially in the needle biopsy setting. The pathologist’s awareness of the vast array of benign mimickers is important in the systematic approach to the diagnosis of prostatic adenocarcinoma. Knowledge of these patterns on routine microscopy coupled with the prudent use of immunohistochemistry will lead to a correct diagnosis and avert a false-positive cancer interpretation.
Modern Pathology (2004) 17, 328–348, advance online publication, 13 February 2004; doi:10.1038/modpathol.3800055
Keywords: prostate gland; adenocarcinoma; mimickers; benign
Prostatic adenocarcinoma is characterized by diverse architectural patterns and as such can be confused with other histological patterns and processes. Histoanatomic structures such as seminal vesicle, inflammatory and reactive conditions and
pathophysiological processes including atrophy,hyperplasia and metaplasia have protean patterns which may simulate adenocarcinoma. Most of these lesions are readily recognized and easily separated from malignancy but they may present problems, especially when dealing with limited sampling in thin core needle biopsies. False-positive cancer diagnosis, albeit uncommon, may be rendered in
some cases leading to serious clinical, psychological and medicolegal consequences. Prostatic biopsy pathology has been identified as a problem area which may lead to litigation.1–3 In my own experience,the most likely patterns giving rise to falsepositive malignant cells are atrophy, post-atrophic hyperplasia, atypical adenomatous hyperplasia (adenosis) and seminal vesicle.
In this paper, the differential diagnosis of prostatic adenocarcinoma will be discussed with emphasis on benign mimickers as outlined in Table 1.Prostatic intraepithelial neoplasia and other carcinomas may be confused with prostatic adenocarcinoma but these topics will not be addressed. The mimickers of other rare prostatic malignancies such as sarcoma will not be covered. The differential
diagnosis of adenocarcinoma has been detailed in several recent reviews and monographs.4–10
A pattern-based approach to differential diagnosis
The utility of the now famous Gleason diagram extends beyond its role as a grading tool.11,12 It is useful in a discussion of the protean architectural patterns that are important in diagnosing prostatic adenocarcinoma. Additionally, it provides a conceptual framework for discussing differential diagnosis.
In the Gleason diagram, there are nine patterns which can be lumped into four major
architectural categories for discussion of differential diagnosis (see Figure 1 and Table 2).The predominant pattern of adenocarcinoma is the small glandular one. This corresponds to Gleason patterns 1, 2, 3A, 3B and consists of separate acini which may be tiny, small or medium in size. Most benign mimickers enter the differential
diagnosis of small acinar adenocarcinoma.The second major pattern is the large glandular one. Medium to large simple acini and/or papillary and cribriform structures are seen. Central (comedo)necrosis involving round duct-like structures may also be identified. The large gland architecture includes Gleason patterns 3A, 3C and 5A.The third major growth pattern of adenocarcinoma is fused glandular which comprises Gleason patterns 4A and 4B. The infiltrating coalesced glands may be either amphophilic (Gleason 4A) or clear (so-called hypernephroid; Gleason 4B). A few
structures and benign processes simulate this fused gland pattern.The final major pattern of adenocarcinoma is the solid one which consists of sheets, cords and single
infiltrating cells and corresponds to a pattern 5B in the Gleason chart. Certain inflammatory lesions may be confused with solid adenocarcinoma.
A benign mimicker may in some situations simulate more than one major pattern of adenocarcinoma. For instance, reactive atypia involving small acini can be mistaken for small acinar carcinoma. However, if the atypia affects medium to large glands, then the differential diagnosis is with the large gland pattern of carcinoma.
The benign mimickers and the corresponding patterns of adenocarcinoma that may be simulated are shown in Table 3. The discussion of these entities will follow this pattern-based approach.
Small Gland Pattern
Seminal vesicle
Seminal vesicle tissue may be present in transurethral resectates or in needle biopsies, usually unexpectedly, but sometimes as a result of specific sampling.13,14 The seminal vesicle is characterized by a central lumen with branching glands surrounded by smooth muscle. Commonly, the end Table 1 Classification of benign mimickers of adenocarcinoma Histoanatomic structures Reactive atypia Seminal vesicle/ejaculatory branching is complex with numerous small glands,resulting in the so-called adenotic pattern of seminal vesicle (Figure 2). This latter pattern can present problems, especially when the overall gland structure and central lumen are not recognized. Tangential sampling of adenotic areas in a needle biopsy may produce a small gland pattern causing confusion with small acinar carcinoma.15 Helpful features to distinguish adenotic seminal vesicle include the presence of nuclear hyperchromasia and pleomorphism which at times is striking.13,16 The degree
of atypia is thought to increase with advancing age.13 Atypical cells are found centrally within the acini and they are often more atypical than the nuclei of small acinar carcinoma (Figure 2). Mitoses are not identified. Small nuclear pseudoinclusions are commonly seen. In the cytoplasm, golden-brown lipofuscin pigment is usually identified, although the amount varies from case to case. When prominent,the diagnosis of seminal vesicle epithelium is easily supported.
It must be remembered however that lipofuscin pigment may be present in normal, hyperplastic,preneoplastic (PIN) and carcinomatous glands.17–19
The presence of lipofuscin pigment in small acinar carcinoma however is rare. When the epithelial atypia and pigmentation are not prominent, a significant diagnostic challenge may result. In problematic cases, negative immunohistochemistry
for prostatic-specific antigen (PSA) and prostatic acid phosphatase (PAP) helpful. Additionally, the 34bE12 stain shows basal cells in seminal vesicular glands which of course are absent in small acinar carcinoma. Ejaculatory duct epithelium has a similar morphology to that of the seminal vesicle. Ejaculatory ducts however are surrounded by a band of loose fibrovascular connective tissue and lack the wellformed
muscular wall of seminal vesicle. This
distinction is of practical importance since the
presence of carcinoma in ejaculatory duct tissue
does not indicate extraprostatic disease whereas
carcinomatous involvement of seminal vesicle proper
indicates high stage disease (at least stage pT3b)
and has adverse prognostic significance.
Cowper’s gland
Cowper’s glands, also referred to as the bulbourethral
glands are paired periurethral structures
located near the prostatic apex.20,21 They are rarely
sampled in prostatic specimens. Cowper’s glands
have a lobular configuration with a central duct
surrounded by tightly packed round acini composed
of cells with abundant mucinous cytoplasm (Figure
3). The nuclei are basally located and uniform.
Sometimes, skeletal muscle, typical of the apical
region, is present in the periglandular stroma.
Cowper’s glands are rarely confused with small
acinar carcinoma. The duct-acinar architecture,
cytoplasmic mucin and lack of cellular atypia
distinguish Cowper’s glands from adenocarcinoma.
In difficult cases, special stains for mucin (mucicarmine,
PAS-D) may be employed. Cowper’s glands
show variable staining results for PSA and are
negative for PAP.20,21 The ductal cells exhibit high
molecular weight keratin (34bE12) staining and in
some cases attenuated cells around the periphery of
the acini are also positive.20 The latter cells may also
exhibit smooth muscle actin positivity.20,21 Another
differential diagnosis of Cowper’s glands is mucinous
metaplasia of prostatic acini which is usually
seen in association with atrophy.22
Atrophy
Atrophy of prostatic glands is a common process
typically but not exclusively found in older patients.
Atrophy may be seen in the young adult prostate
and is commonly admixed with areas of nodular
prostatic hyperplasia.23 While common in the
peripheral zone, atrophy may also be seen in the
central and transition zones. Glandular atrophy is
commonly associated with chronic prostatitis which
may have an active component characterized by
intraglandular neutrophils. Some recent evidence
suggests that prostatic atrophy may be a manifestation
of chronic ischemic disease, although many
examples of atrophy are still considered idiopathic
in nature.24 Atrophy can also be the result of
treatment with radiation and antiandrogens25–30
(Figure 4). Treatment-associated atrophy is frequently
extensive and severe and in the case of
radiation may be associated with epithelial atypia.
Four main patterns of atrophy are recognized—
lobular (simple), sclerotic, cystic and linear or
streaming (Figure 5). Combined patterns are common.
Lobular (simple) atrophy is characterized by
small glands arranged in circumscribed (lobular)
nests. The orderly architecture is best appreciated
on low power. In sclerotic atrophy, the small glands
are distorted by dense collagenized stroma which
results in sharply angulated and irregular shapes.
The stroma often has an elastotic appearance similar
to that seen in some breast conditions. At low
power, the lobular architecture is usually evident
and sometimes a central dilated duct is appreciated.
The almost desmoplastic appearance of the stroma
in sclerotic atrophy contrasts with low grade, small
acinar carcinoma which incites little or no stromal
response. In cystic atrophy, varying degrees of acinar
dilatation are seen and usually other areas of more
typical simple atrophy are found nearby. Occasionally,
atrophy may have a linear, streaming pattern in
which small dark acini are lined up in a row,
seemingly permeating through stroma. This latter
pattern is particularly prone to be overinterpreted as
carcinoma.
Regardless of the architectural subtype of atrophy,
the cytological features are similar. The cells are
small, shrunken and dark. They have high nuclear to
cytoplasmic ratios but the nuclei are uniform and
lack nuclear membrane irregularity and chromatin
abnormalities. Occasionally, small chromocenters
are seen but prominent nucleoli are absent. Double
layering of cells is often seen but in some instances
it may be difficult to appreciate because of the
marked secretory cell atrophy (Figure 6). In such
cases, stains for high molecular weight keratin
(34bE12) may be employed to highlight the basal
cell compartment.31
The important features in separating atrophy from
small acinar carcinoma are the low power maintenance
of lobular architecture at least in part,
uniform cytology and absence of prominent nucleoli.
In some cases, especially when the atrophy is
along the edge of a biopsy or when distortion or
secondary inflammation is present, diagnosis may
be difficult and special stains for high molecular
weight keratin should be employed.
In a series of secondary reviews of prostatic
pathology prior to definitive treatment, atrophy
was a lesion in needle biopsies sometimes
overinterpreted as adenocarcinoma.32 It should
be emphasized that carcinoma may have an
atrophic appearance and as such may be underinterpreted
as atrophy. Atrophic adenocarcinomas
are composed of small dark acini usually with
an irregular permeative pattern of growth. On
close inspection, there is nuclear atypia and nucleoli
are usually recognized at least focally. In some
instances, 34bE12 staining may be required
to distinguish the atrophic adenocarcinoma from
atrophy.33,34
Atrophy associated with inflammation, especially
active inflammation, may be particularly challenging
and one should be cautious in diagnosing
carcinoma in inflamed small gland foci. Additionally,
in the setting of radiation, atrophy may be
severe and may be associated with stromal
fibrosis. This may result in architectural distortion
which when coupled with cytological atypia
typical of radiation effects can cause considerable
diagnostic confusion. In such cases, high molecular
weight keratin stains (34bE12) are useful to
separate non-neoplastic atrophic glands from
residual carcinoma.
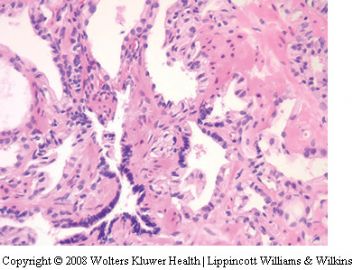
Post-atrophic hyperplasia
Post-atrophic hyperplasia, also referred to as partial
atrophy or hyperplastic atrophy is an uncommon
histological process found in about 2–3% of prostatic
needle biopsy cases.35–38 It is usually found in
the peripheral zone. In most cases, it is difficult to
know if post-atrophic hyperplasia represents a
normal or hyperplastic focus undergoing atrophy
(partial atrophy) or secondary hyperplasia occurring
in atrophic areas. Studies with proliferation markers
however show increased proliferation in areas of
post-atrophic hyperplasia, thus supporting the latter
hypothesis.39,40
Post-atrophic hyperplasia consists of a combination
of atrophic acini and ones that contain more
abundant clear or amphophilic cytoplasm and
appear hyperplastic (Figure 7). The lobular arrangement
is usually maintained and there is often
apparent budding of ‘neoacini’ lined by cuboidal
cells with clear cytoplasm. A mixture of cells with
atrophic and nonatrophic cytoplasm leads to a
variety of irregular glandular shapes including
stellate ones. Some nuclear enlargement may be
seen and rarely, enlarged nucleoli are identified. The
busy architecture of post-atrophic hyperplasia may
cause diagnostic confusion with adenocarcinoma,
however there is generally a maintenance of some
degree of lobular architecture and basal cells are
usually recognized, even at the H&E level. Caution
should be exercised whenever there is an admixture
of atrophic and nonatrophic acini, especially when a
lobular pattern is not readily recognized. Prominent
nucleoli in a significant number of cells are not
typically found in post-atrophic hyperplasia. The
prudent use of high molecular weight keratin
(34bE12) immunostains is important in difficult
cases. There is a discontinuous layer of basal cells
in post-atrophic hyperplasia whereas in small acinar
carcinoma, the basal cell layer is completely absent.
In rare instances, luminal crystalloids and even
small amounts of basophilic luminal mucus may be
present in post-atrophic hyperplasia.
The early ideas of Franks and Laivag that atrophy
and post-atrophic hyperplasia are involved in the
pathogenesis of prostatic cancer were largely ignored
until recently.35,39 The common topographic
association of prostatic carcinoma, atrophy and
chronic inflammation is well known but most
investigations had been focused on other putative
precursors such as prostatic intraepithelial neoplasia
(PIN) and atypical adenomatous hyperplasia
(adenosis). De Marzo and co-workers have recently
identified a lesion which they refer to as proliferative
inflammatory atrophy which may represent a
precursor of adenocarcinoma.40–42 This lesion is
morphologically represented by areas of simple
atrophy and/or post-atrophic hyperplasia in which
there is superimposed chronic inflammation. Using
immunohistochemistry, the above authors have
identified areas of high cell proliferation in which
there is increased staining of P-glutathione stransferase
(GSTP1) and bcl-2 along with decreased
staining of the cyclin-dependent kinase inhibitor
p27. The findings suggest that the cells of the
secretory compartment in proliferative inflammatory
atrophy have an immature secretory phenotype
similar to that seen in cells of high-grade PIN and
carcinoma. The authors have identified areas of
atrophy merging into high-grade PIN within the
same glands. They suggest that proliferative inflammatory
atrophy may give rise to carcinoma, either
directly or through high-grade PIN as an intermediary
step. Clearly, further investigations are required
to evaluate this hypothesis. It should be stressed
however that proliferative inflammatory atrophy is a
lesion which is not defined solely on the basis of the
H&E morphology but requires immunohistochemical
markers of proliferation and differentiation for
identification.
Reactive atypia
Epithelial atypia may be seen in association with
acute or chronic prostatitis and may sometimes be
present in association with prostatic ischemia
(infarction). Reactive atypia can be confused with
adenocarcinoma43–45 (Figure 8). The glands in most
cases of reactive atypia are atrophic and there may
be some associated basal cell or transitional cell
hyperplasia. In some cases, especially in ischemia,
the epithelium may have a squamoid appearance
and frank squamous or transitional metaplasia may
be present (Figure 9). Mild to moderate nuclear
enlargement is seen and sometimes nucleoli are
prominent. The nucleolar enlargement may actually
exceed that of adenocarcinoma.
The low-power architecture, presence of basal
cells and degree of cytological atypia usually allow
separation of reactive lesions from adenocarcinoma.
However, adenocarcinoma may be present in areas
of prostatitis and ischemia and as such diagnostic
caution should prevail.
A special situation of reactive atypia is radiation
effects.26,27 The degree of cytological atypia in
irradiated prostates may be severe with enlarged,
hyperchromatic nuclei and prominent nucleoli. The
low-power architectural arrangement of the atypical
glands is helpful in separating radiation atypia from
residual adenocarcinoma. Other radiation-associated
changes include prominent atrophy, transitional
cell and squamous metaplasia, stromal
fibrosis, edema and changes within prostatic arteries.
In difficult cases, immunohistochemistry for
high molecular weight keratin (34bE12) is invaluable
in resolving the differential diagnosis.
Mucinous metaplasia
Mucin-producing cells are sometimes identified
within prostatic glands, usually atrophic ones.22 As
such, this process may enter the differential diagnosis
of small acinar carcinoma. The lobular pattern
of the associated atrophy usually points to the
benign nature of this lesion (Figure 10). Individual
mucous cells have a vacuolated appearance on H&E
and highlighted by mucicarmine and PAS stains.
When there is florid mucinous metaplasia, especially
in an apical location, confusion with Cowper’s
(bulbourethral) glands may occur.20,21
Nephrogenic metaplasia (adenoma)
Nephrogenic metaplasia (adenoma) is uncommonly
encountered in the prostatic urethra, and subjacent
prostatic tissue.46–49 It may be present as an
exophytic (papillary) lesion or as a flat or nodular
abnormality. In most instances, there has been a
previous history of trauma, instrumentation or
transurethral resection. Problems can arise when a
prior transurethral resection has revealed adenocarcinoma.
Nephrogenic metaplasia displays exophytic papillary
and tubulocystic elements. The latter may
have a pseudoinvasive growth pattern (Figure 11).
The acini of nephrogenic metaplasia are usually
small and the cells have scant cytoplasm. Sometimes
they may have more abundant clear cytoplasm.
At least focally, the tubules may display
cystic change and sometimes hobnail cells are
identified. Commonly, the adjacent stroma is both
edematous and inflamed.
The small size of the acini, cystic dilatation and
inflamed stroma separate nephrogenic metaplasia
from small acinar carcinoma. High molecular weight
keratin (34bE12) is positive in some but not all cases
of nephrogenic metaplasia.49 When positive, it is a
helpful adjunctive test. The PSA and PAP stains are
usually negative but there may be focal positivity of
tubular cells and/or secretions.49
A recent study suggests that nephrogenic metaplasia
(adenoma) is neither metaplastic nor neoplastic
in nature.50 Molecular studies in the setting of
transplantation suggest that this lesion is truly
nephrogenic in origin and results from the implantation
of renal tubular cells at sites of prior
urothelial injury with subsequent proliferation of
epithelial elements.
Basal cell hyperplasia
Basal cell hyperplasia is typically seen as part of the
spectrum of nodular hyperplasia usually in samples
from the transition zone.51–54 Recently, it has been
recognized that basal cell hyperplasia may also
affect the peripheral zone.55 It is usually identified
in transurethral resection specimens but may be
encountered in needle biopsies (Figure 12). Basal
cell hyperplasia may also occur in association with
atrophy, usually in the setting of antiandrogen
therapy.28–30 Basal cell hyperplasia may be confused
with adenocarcinoma.
Basal cell hyperplasia is usually characterized by
nodular expansion of uniform round glands associated
with a cellular stroma. It may be complete or
incomplete.15 There is a lack of secretory (luminal)
cell differentiation in the complete form in which
solid nests of dark-blue cells are present. In the
incomplete form, there are residual small lumina
lined by secretory cells with clear cytoplasm and
these are surrounded by multiple layers of basal
cells. In each type, the basal cells are dark and have
scant cytoplasm and display round, oval or somewhat
spindled hyperchromatic nuclei (Figure 12).
Nucleoli are usually indistinct but in some
examples of the so-called atypical basal cell hyperplasia,
nucleoli may be more prominent.53,56 Microcalcifications
are present in up to half of the cases of
basal cell hyperplasia. The adjacent stroma is often
hypercellular and consists of proliferating fibroblasts
and smooth muscle cells similar to those seen
in usual nodular hyperplasia.
Basal cell hyperplasia is readily separated from
adenocarcinoma in most cases, especially in transurethral
resectate and prostatectomy specimens.
The nodular arrangement, association with ordinary
nodular hyperplasia, cellular uniformity and lack of
prominent nucleoli serve to separate this condition
from cancer. It may be more difficult however in
small biopsies in which part of a focus of basal cell
hyperplasia is only partially represented. In such
cases, the distinction relies on the identification of
uniform cytological and nuclear features and in
some instances may require immunohistochemical
staining for high molecular weight keratin
(34bE12).52
Benign nodular hyperplasia, small gland pattern
Benign nodular hyperplasia of prostate can have a
variety of morphologies including predominantly
stromal, mixed glandular and stromal, and predominantly
glandular growth patterns.9,15 The glandular
elements usually consist of medium to large
acini, often showing luminal papillae. Occasionally,
areas of nodular hyperplasia are composed of
mainly small to medium sized, closely packed acini
with rounded lumens.
Usually basal cells are readily identified and
small amounts of intervening cellular stroma are
seen. The nodular circumscription, uniform architecture,
presence of basal cells and intervening
stroma serve to separate this proliferative pattern
from low-grade (Gleason patterns 1, 2) adenocarcinoma
(Figure 13).
It is probably more common for adenocarcinoma
to mimic benign nodular hyperplasia than the
converse. The pseudohyperplastic pattern of adenocarcinoma
is composed of medium to large acini
that may have a somewhat nodular appearance on
low power.57,58 This form of carcinoma can be
deceptively bland and may only be suspected when
there is a subtle disruption of the normal gland/
stroma relationship on low power. This observation
can be quite difficult in thin core biopsies. On
higher power, there is an absence of basal cells and
the typical nuclear features of adenocarcinoma are
present. The cells comprising this lesion are usually
high cuboidal to columnar cells with abundant
apical, often clear cytoplasm. These cells resemble
the appearance of the secretory cell compartment in
benign nodular hyperplasia aside from their nuclear
features. The 34bE12 stain is invaluable in confirming
a diagnosis of pseudohyperplastic adenocarcinoma.
Sclerosing adenosis
The term sclerosing adenosis of the prostate was
first used by Young and Clement in 1987 to describe
an unusual prostatic proliferative lesion which
resembled to some extent sclerosing adenosis of
breast.59 There had been an earlier report in which
the term adenomatoid tumor was used.60 Sclerosing
adenosis is an uncommon lesion largely restricted to
the transition zone and is generally an incidental
finding in transurethral resectates or radical prostatectomy
specimens. It is rarely seen in needle
biopsies. A few series of sclerosing adenosis cases
have been reported.61–63
Sclerosing adenosis is characterized by a more or
less circumscribed proliferation of variably sized,
often small glands embedded in a cellular and often
edematous stroma (Figure 14). Sometimes the lightly
basophilic stroma is recognized on low power. Tiny
microacini, cords, solid clusters and single cells are
seen. A double layer is present but may be difficult
to appreciate with the H&E stain. The lining cells
often have open nuclear chromatin with inconspicuous
nucleoli although prominent nucleoli may be
focally present. Glandular lumens may contain
crystalloids or occasionally acid mucin. A characteristic
feature is the present of a thick eosinophilic
basement membrane around at least some glands.
Sclerosing adenosis is unique in that the basal
cells in this condition undergo myoepithelial metaplasia
and show coexpression of both high molecular
weight cytokeratin and muscle-specific actin
(HHF-35)63 (Figure 14). S100 protein is also positive
in these cells. The myoepithelial differentiation has
been confirmed by electron microscopic studies.
The key features distinguishing sclerosing adenosis
from adenocarcinoma include the variation in
gland size and shape, thickened basement membranes
and cellular stroma. Immunohistochemistry
for high molecular weight keratin (34bE12) and actin
can be used in problematic cases.
Verumontanum mucosal gland hyperplasia
Verumontanum mucosal gland hyperplasia is identified
as an incidental finding in radical prostatectomy
specimens.64 In all, 14% of 30 radical
prostatectomy specimens in one series contained
one or more foci of hyperplastic verumontanum
mucosal glands. This process is rarely encountered
in needle biopsy specimens.65 It is characterized by
relatively uniform, closely packed, round glands
containing numerous corpora amylacea which may
have a red or orange-brown coloration (Figure 15).
Basal cells are usually identified and there is a lack
of nuclear features of malignancy. In particular,
prominent nucleoli are not seen. Lipofuscin pigment
may be present within the cytoplasm of
glandular cells. Prostatic urethral tissue is often seen
nearby and islands of transitional cell epithelium
may be present in or adjacent to the proliferating
verumontanum glands. The suburethral location,
acinar and cellular uniformity, basal cells and
prominent corpora amylacea allow separation of
this entity from low-grade adenocarcinoma. Recently
verumontanum mucosal gland hyperplasia
has been associated with atypical adenomatous
hyperplasia in a radical prostatectomy series.66
Hyperplasia of mesonephric glands
Mesonephric gland remnants are rarely identified
in prostatic specimens.67–71 In a series of close to
700 transurethral resectates, 0.6% contained mesonephric
remnants.70 Mesonephric gland remnants
occasionally undergo hyperplasia and may be
confused with adenocarcinoma. Gikas et al identified
two cases in transurethral resection specimens
that were interpreted as adenocarcinoma which
in one instance led to an unnecessary radical
prostatectomy.67 The hyperplastic mesonephric
glands are typically small and may have an
infiltrative appearance (Figure 16). Sometimes
tubular dilatation, epithelial tufting and micropapillary
formations are seen. They may demonstrate
perineural spread and extraprostatic extension.
Mesonephric glands are lined by a single layer
of cuboidal cells. Typically, the small glands contain
a dense eosinophilic luminal substance which
contrasts with the loose granular eosinophilic
material typical of small acinar carcinoma (Figure
16). Immunohistochemistry may be helpful in
difficult cases. The glands of mesonephric hyperplasia
stain negatively for PSA and PAP and often
positively for high molecular weight cytokeratin
(34bE12) in contrast to adenocarcinoma which lacks
34bE12 basal cells.
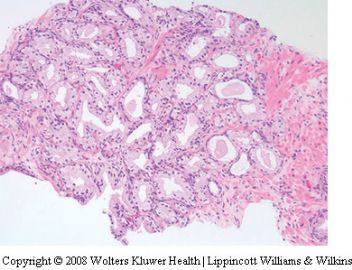
Atypical adenomatous hyperplasia (adenosis)
Atypical adenomatous hyperplasia (AAH) is a
proliferative lesion characterized by crowded small
acini, usually forming well circumscribed nodules
which often simulate the small gland pattern of
carcinoma.72–78 While many authors use the term
Figure 15 Verumontanum mucosal gland hyperplasia. (a) Lowpower
appearance in needle biopsy. (b) High-power appearance
showing tightly packed small acini with prominent corpora
amylacea.
‘atypical adenomatous hyperplasia’ others prefer
the rubric ‘adenosis’.79–83 AAH is a well-defined
entity but the term ‘adenosis’ has been used more
loosely, even to the extent that some examples of
adenosis have been interpreted by experts as
adenocarcinoma.84
AAH has been identified in 1.5–19.6% of transurethral
resectates and in up to 33% of radical
prostatectomy specimens.9 It is uncommon in
needle biopsy specimens but occasionally occurs.82
Most evidence associating AAH with carcinoma is
circumstantial.9,75,78 AAH has a predilection for the
transition zone and morphologically simulates low
grade (Gleason 1, 2) carcinoma. Examples of small
acinar carcinoma arising in relationship to AAH
have been reported. Additionally, the age of patients
with AAH is usually 5–10 years, less than those
with carcinoma.
The basal cell-specific keratin stain shows a
discontinuous pattern which is intermediate between
the continuous pattern of normal prostate and
the absence of basal cells in carcinoma.8,74,85 Studies
with tridiated thymidine, immunohistochemistry
for proliferation markers (Ki67/M1B-1) and silver
staining of nucleolar-organizer regions suggest that
AAH has a proliferation rate between benign
prostate hyperplasia and low-grade carcinoma.86–88
Recent molecular and phenotypic studies suggest a
possible linkage between AAH and carcinoma in the
minority of cases.89,90
While circumstantial evidence exists, there is lack
of proof of a relationship between AAH and
adenocarcinoma.9 It has been suggested that AAH
is a precursor of some low-grade transition zone
carcinomas but the lack of an increased prevalence
of AAH in prostate glands with transition zone
carcinoma argues against this hypothesis.9 Clearly,
there is less evidence linking AAH to carcinoma
than there is for high-grade PIN and cancer.9,75
The major importance of AAH is its potential
for being misdiagnosed as adenocarcinoma. Foci
of AAH are usually less than 5mm across and
are characterized by a proliferation of relatively
small uniform acini, often within or adjacent to
typical hyperplastic nodules (Figure 17). Sometimes
there is a prominent perinodular distribution
of the abnormal glands. The low-power architecture
is reminiscent of Gleason patterns 1 and 2 carcinoma.
AAH usually has a pushing rather than
infiltrating border but may show a limited degree
of infiltration (Figure 18). Individual glands
are closely packed but separate and show no
evidence of fusion. They show some variation in
size and shape and are lined by cuboidal to low
columnar cells with moderate to abundant clear or
lightly eosinophilic cytoplasm. Basal cells are
usually recognized at least focally. The luminal
borders are often irregular and somewhat serrated
in contrast to the rigid borders that typify small
acinar carcinoma. The lumens are often empty
but may contain corpora amylacea and in some
instances luminal eosinophilic crystalloids.74,81
Occasionally, basophilic luminal mucus may be
seen.91,92 There is usually no stromal response but
occasionally, a fibroblastic response is identified
which leads to overlap with the pattern of sclerosing
adenosis.59–63
The nuclei of atypical adenomatous hyperplasia
are round to oval and there is uniform fine
chromatin.74 Nucleoli may be present but they are
generally small. Uncommonly, enlarged nucleoli
(41 mm) are identified in a subset of cells.74
By immunohistochemistry, the glands of AAH
exhibit strong positivity for PSA and PAP and there
is typically a discontinuous basal cell pattern with
the 34bE12 stain (Figure 17).
The most important features in separating AAH
from adenocarcinoma is the lack of significantly
enlarged nucleoli and the presence of a fragmented
basal cell layer. A comparison between high- and
low-grade carcinoma is shown in Table 4.
From a clinical perspective, AAH should be
considered as a benign lesion and patients followed
conservatively. The term should not be used as a
‘wastebasket’ for small glandular lesions that are
difficult to classify or for suspicious atypical small
gland proliferations (atypical small acinar proliferation
[ASAP], glandular atypia), just below the
threshold of adenocarcinoma.9
Large Gland Pattern
Clear cell cribriform hyperplasia
Benign nodular hyperplasia occasionally displays
areas of prominent cribriform glands. Rarely, the
cribriform process dominates the histologic picture.
93,94 Cribriform hyperplasia is characterized by
a crowded proliferation of complex glands without
cytologic atypia. In most instances, the cribriform
glands have clear cytoplasm and uniform round
lumina. This lesion generally has a low-power
nodular appearance and intervening cellular stroma
is seen (Figure 19). The cells comprising the central
cribriform areas are cuboidal to low columnar
secretory-type cells with uniform round nuclei and
clear cytoplasm. They lack nuclear atypia and
nucleolar enlargement. Basal cells are prominently
displayed around the periphery.
Cribriform hyperplasia enters the differential
diagnosis of both prostatic intraepithelial neoplasia
and cribriform adenocarcinoma. The distinction of
cribriform hyperplasia from cribriform carcinoma is
based on the ‘low power’ nodularity, cellular stroma,
presence of basal cells and lack of significant
cytologic atypia.
Adenoid cystic-like basal cell hyperplasia
While most forms of basal cell hyperplasia are
characterized by relatively small nests of basal cells,
the incomplete form of basal cell hyperplasia may be
composed of medium to large glands with a complex
cribriform pattern, sometimes with cyst formation
and squamous metaplasia.9,52,95,96 Such a process
rarely enters the differential diagnosis of cribriform
carcinoma. Some of these cases have been termed
adenoid basal cell tumor or even basal cell carcinoma
(Figure 20). The presence of typical areas of basal
cell hyperplasia, lack of significant infiltration
and absence of cytological atypia favor adenoid
cystic-like basal cell hyperplasia over cribriform
carcinoma.
Reactive atypia in large glands
Medium to large glands may display reactive atypia
in the setting of inflammation, ischemia and radiation.
9,43–45 Such processes may lead to glandular
distortion and nuclear atypia which sometimes
results in a pattern that may be confused with
prostatic intraepithelial neoplasia (PIN) and large
gland patterns of adenocarcinoma. The most helpful
features to distinguish reactive atypia from
malignancy is the recognition of the associated
inciting factor such as inflammation, infarction
or radiation, and the maintenance of the basal
cell compartment. The atypia associated with
reactive conditions may result in nuclei that
appear hyperchromatic and somewhat degenerate.
In some cases, the nucleolar enlargement associated
with the reactive state may be more prominent
and more uniform than that seen with
adenocarcinoma. The low-power architecture and
presence of a residual basal cell layer sometimes
requiring confirmation with the 34bE12 stain
are the best clues to the benign nature of this
condition.
Fused Gland Pattern
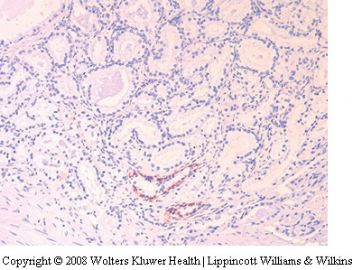
Paraganglion
Paraganglionic tissue may be encountered within
prostatic and periprostatic tissue, usually the latter.
97–99 Paraganglia are characterized by small, solid
nests of cells with clear or amphophilic cytoplasm,
often with a ‘zellballen’ arrangement. There is a
delicate background network of capillaries. The
nuclei are often hyperchromatic but nucleoli and
other features of adenocarcinoma are not seen. The
islands of paraganglionic tissue are separated by
fibrous stroma. Paraganglionic tissue can simulate
the fused gland pattern of adenocarcinoma (Gleason
4) (Figure 21). If the cytoplasm of the paraganglionic
tissue is amphophilic, it may look like Gleason
pattern 4A and if clear may look like Gleason pattern
4B. Cases in needles biopsies are quite rare. A more
common issue is the overinterpretation of extraprostatic
paraganglionic tissue in radical prostatectomy
specimens leading to spurious overstaging of organconfined
cancer. Additionally, the interpretation of
paraganglionic tissue as adenocarcinoma may lead
to a grading inaccuracy. When a Gleason grade 3
tumor is encountered and accompanying paraganglionic
tissue is interpreted as Gleason grade 4
tumor, the resultant score would be inaccurately
Figure 20 Adenoid cystic-like basal cell hyperplasia. (a) Lowpower
photomicrograph showing a rounded collection of large
basaloid nests embedded in loose stroma. (b) Medium-power
photomicrograph showing proliferation of basaloid nests and
cribriform structures with focal dense amorphous material.
recorded as 7 instead of the correct score of 6. In
problematic cases, special stains determining prostatic
origin (PSA, PAP) and stains for neuroendocrine
cells (chromogranin, synaptophysin) should
be employed (Figure 21).
Xanthogranulomatous prostatitis (xanthoma)
Collections of lipid-laden macrophages in the
prostate may cause diagnostic confusion with the
hypernephroid pattern of adenocarcinoma (Gleason
4B)100,101 (Figure 22). Xanthomatous histiocytes
usually have small uniform nuclei within inconspicuous
nucleoli and are commonly admixed with
other types of inflammatory cells. In some instances,
there is almost a pure population of foam cells
which can lead to considerable diagnostic confusion.
The problem is compounded by the fact that
some hypernephroid carcinomas do not show the
typical nuclear features of malignancy. They may
have small dark nuclei without prominent nucleoli.
In rare cases, immunohistochemistry utilizing stains
for epithelial and prostatic cells (cytokeratin, PSA,
PAP) and histiocytes (CD68) is required to resolve
the diagnostic confusion.
Malakoplakia
Malakoplakia of the prostate is a rare infiltrative
lesion characterized by diffuse sheets of histiocytes,
usually admixed with other inflammatory cells
including lymphocytes, plasma cells and neutrophils.
102–106 In the early phase of malakoplakia when
von Hansemann histiocytes predominate, the lesion
may simulate carcinoma, especially Gleason pattern
4B. The lack of any acinar differentiation and
admixed inflammatory infiltrate along with the
typical Michaelis–Gutmann bodies will lead to a
correct diagnosis (Figure 23). The absence of
cytokeratins and prostatic epithelial markers along
with the presence of CD68 staining may resolve
difficult diagnostic problems.
Solid Pattern
Ordinary prostatitis
Occasionally, needle biopsies with prostatitis of the
usual type may cause diagnostic problems.107–109
This is especially true when there is poor preservation
and mechanical (crush) artifacts (Figure 24). In
some cases, immunohistochemical stains (keratins,
Figure 21 Prostatic paraganglion. (a) Needle biopsy showing
solid amphophilic area. (b) High-power photomicrograph showing
rounded amphophilic cellular mass without significant
atypia. (c) Positive chromogranin stain.
leukocyte common antigens) are required in order to
resolve a differential diagnosis.
Non-specific granulomatous prostatitis
Granulomatous prostatitis commonly results in a
prostate gland that feels firm to hard and clinically
simulates carcinoma.110,111 In biopsy samples,
especially needle biopsies, florid nonspecific
granulomatous prostatitis may simulate carcinoma.
112,113 The association of the inflammation
with ducts may not be seen and when the
inflammatory process is diffuse, it may raise the
suspicion of high-grade (Gleason 5) carcinoma
(Figure 25). The problem is amplified if poor
preservation or mechanical artifacts are present.
The recognition of the inflammatory nature of
the cells along with the association of giant cells
and fibrosis are helpful features. In difficult
cases, immunohistochemistry for cytokeratins,
prostatic epithelial markers and lymphohistiocytic
markers may be used (Figure 26). Granulomatous
prostatitis associated with specific infections
such as fungus and tuberculosis, BCG-associated
granulomas and the procedural associated granulomas
(palisading granulomas) do not usually
cause problems in the differential diagnosis of
adenocarcinoma.
Degenerative changes in lymphocytes and stromal
cells
Lymphocytes and sometimes stromal cells may
undergo degenerative changes which result in a
signet ring-like morphology.114–116 If the change is
prominent, the pattern can resemble high-grade
adenocarcinoma composed of individual signet ring
cells (Figure 27). The artifactual signet ring-like
pattern, while initially described in transurethral
resectates, may be found in needle biopsies. In the
series of 47 cases, these ‘atypical’ cells were
prominent in three cases and rare in 11 cases.115 It
is very important to be aware of this phenomenon
and not to overinterpret such cells as being
malignant. In difficult cases, immunohistochemical
stains can be used to confirm the nonepithelial
nature of the cells.
Summary
There are a wide variety of patterns and processes
that may be confused with one or more of the
diverse patterns of prostatic adenocarcinoma. In
general, recognition of this differential diagnosis
coupled with careful routine microscopy will
lead to a correct diagnosis. In some instances
however, ancillary immunohistochemical studies
aimed at identifying prostatic basal cells (34bE12,
CK5/6, p63), prostatic secretory cells (PSA,
PAP, CD57), neuroendocrine cells (chromogranin,
synaptophysin) and inflammatory cells (LCA,
CD68) may be required to resolve a diagnostic
dilemma (see Table 5). The new marker a-methylacyl-
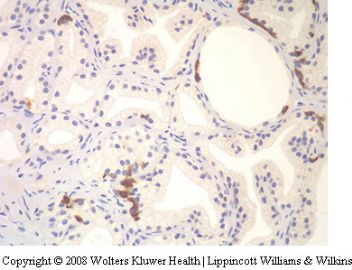
CoA racemase (P504S) appears to be of value
in supporting a diagnosis of adenocarcinoma,
especially when one is dealing with small foci117,118
(Figure 28).
Awareness of the differential diagnosis of prostatic
adenocarcinoma is especially important in the
context of diagnosing limited carcinoma in small
biopsy samples. It is important to always be aware of
the potential of false-positive cancer diagnosis when
looking at prostatic biopsies and to utilize appropriate
consultation and ancillary studies to arrive at
a confident and correct diagnosis.
Figure 26 Granulomatous prostatitis (a) Immunohistochemistry
for cytokeratin (CAM 5.2)—note positive glands (upper right) and
negative staining of infiltrate. (b) Infiltrate stains positively for the
macrophage marker, CD68.
Figure 27 Degenerative changes in lymphocytes and stromal cells
simulating signet ring pattern of prostatic adenocarcinoma.
Table 5 Benign mimickers of adenocarcinoma: useful immunohistochemical
markers
Cytokeratins (general)
AE1/AE3, Cam 5.2, MAK6
Basal cell markers
34bE12, CK5/6, p63
Secretory cell markers
Prostate-specific antigen (PSA), prostatic acid phosphatase
(PAP), CD57
Neuroendocrine markers
Chromogranin, synaptophysin
Lymphohistiocytic markers
Leukocyte common antigen, CD56, CD68
Other markers
a-Methylacyl-CoA racemase (P504S)
Acknowledgements
We gratefully acknowledge the photographic assistance
of David Charters and the excellent secretarial
support provided by Barbara Jones.
Benign mimickers of prostatic adenocarcinoma
JR Srigley
348
Modern Pathology (2004) 17, 328–348
.........
.........
先放一个查到的文献这里,慢慢学习鉴别要点。
可能主要从年龄、部位、形态学(组织结构、细胞学)、免疫组化等方面。
感谢楼主的病例和老师们的提示和启发。