| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
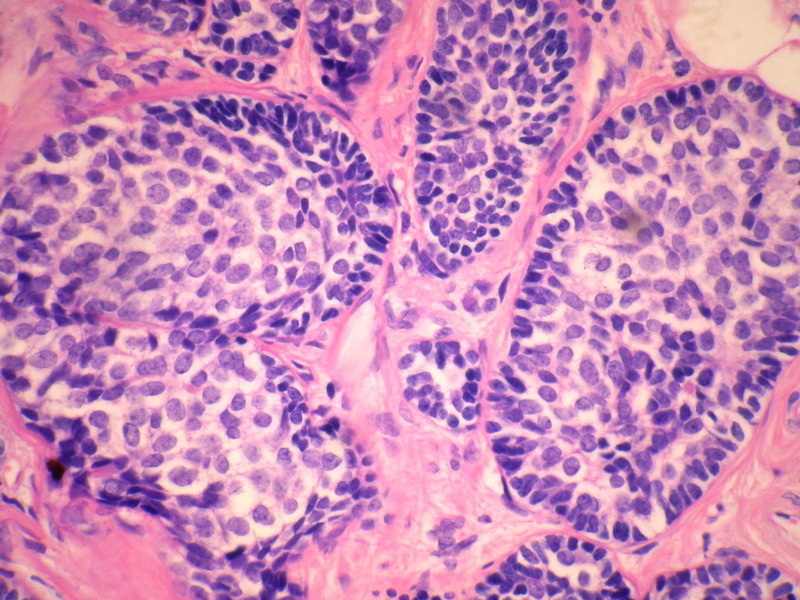
- B228652岁女性,乳腺癌,求类型。已发免疫组化。
| 姓 名: | ××× | 性别: | 年龄: | ||
| 标本名称: | |||||
| 简要病史: | |||||
| 肉眼检查: | |||||
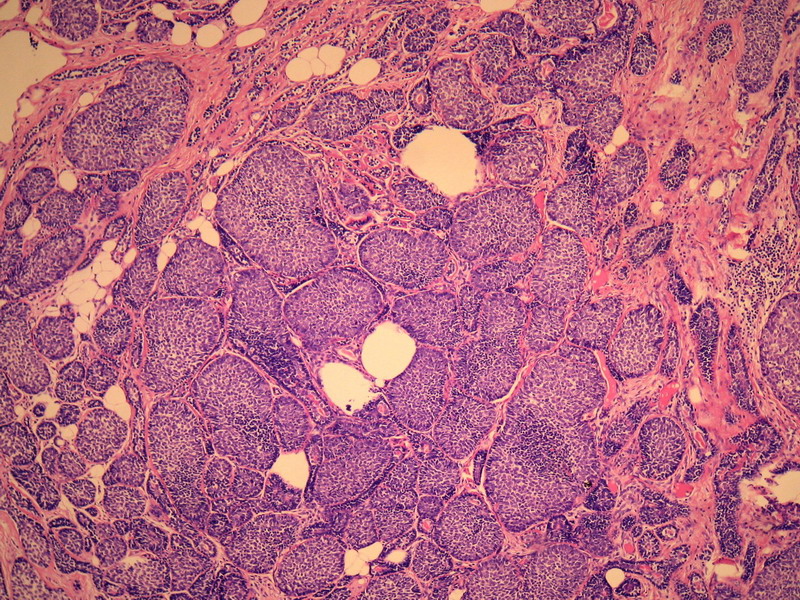
名称:图1
描述:图1
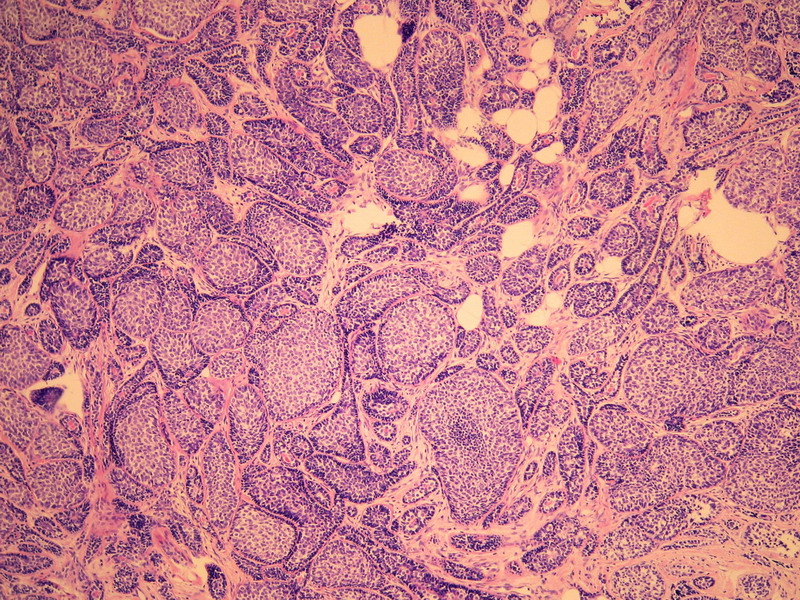
名称:图2
描述:图2
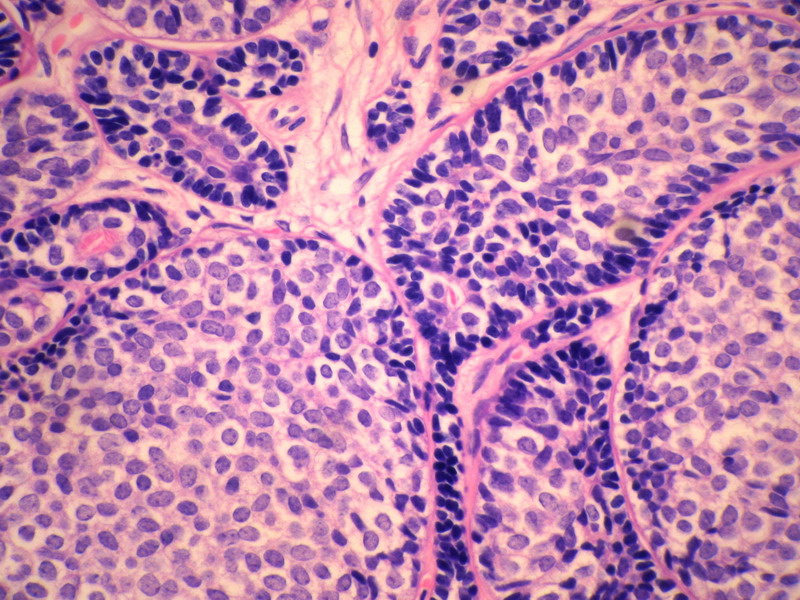
名称:图3
描述:图3
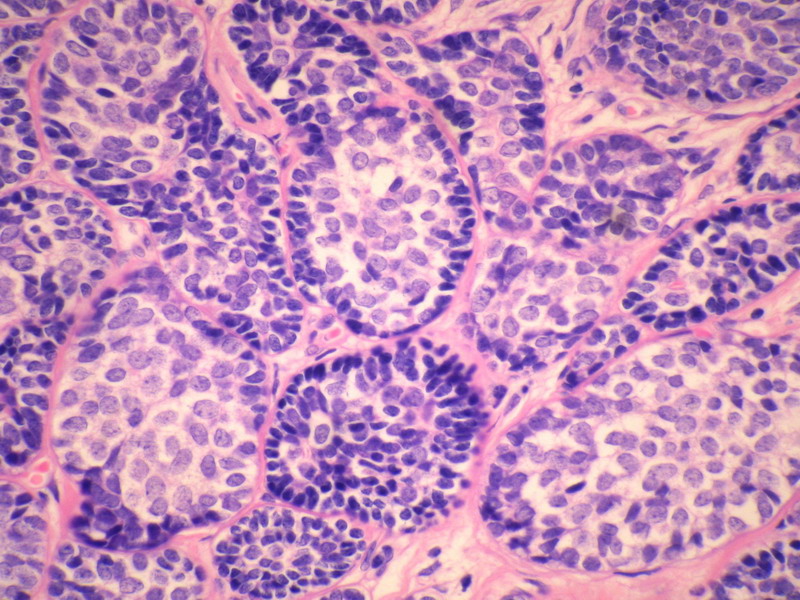
名称:图4
描述:图4
名称:图5
描述:图5
名称:图6
描述:图6
名称:图7
描述:图7
名称:图8
描述:图8
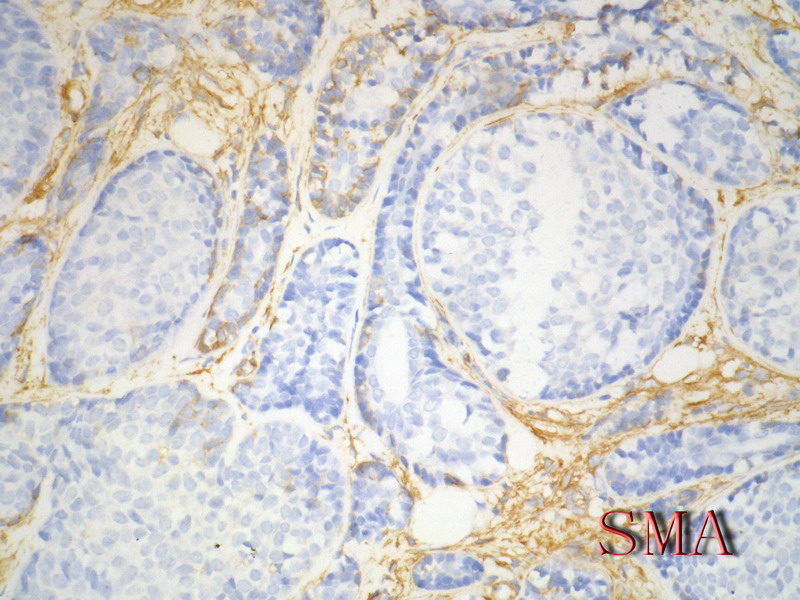
名称:图9
描述:图9
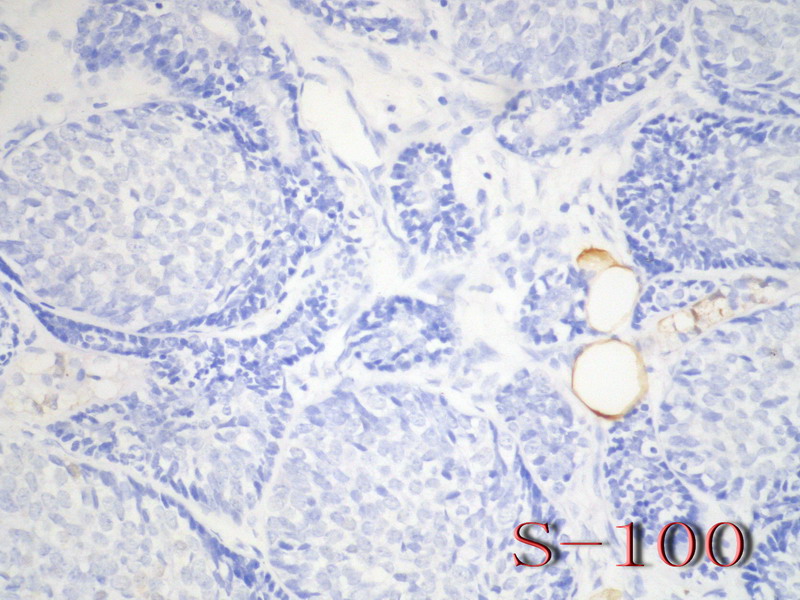
名称:图10
描述:图10
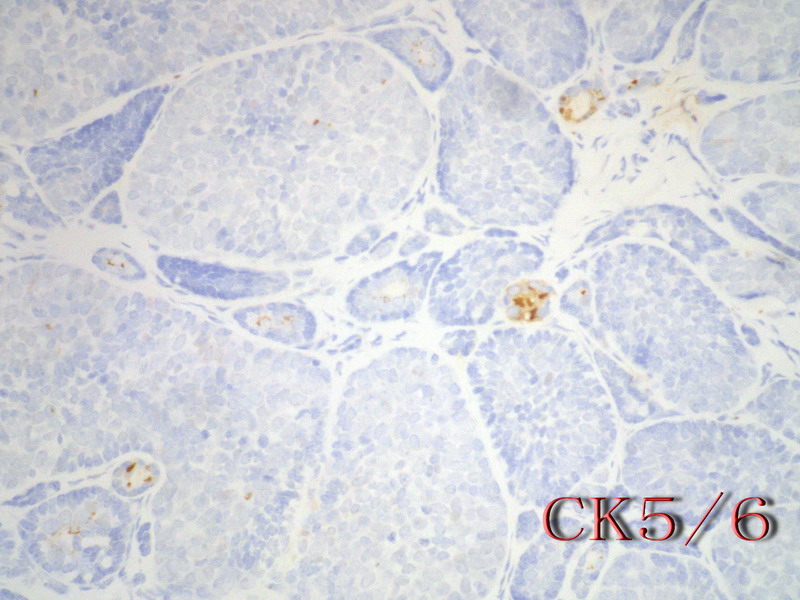
名称:图11
描述:图11
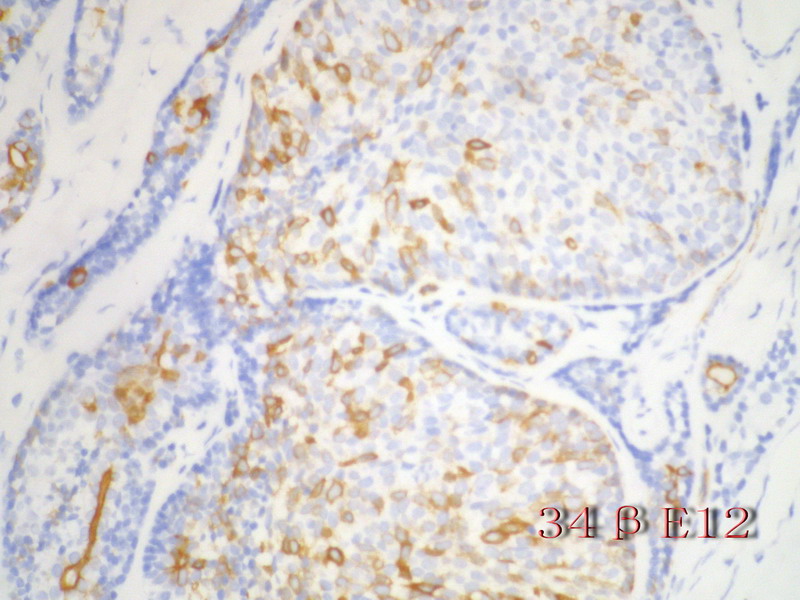
名称:图12
描述:图12
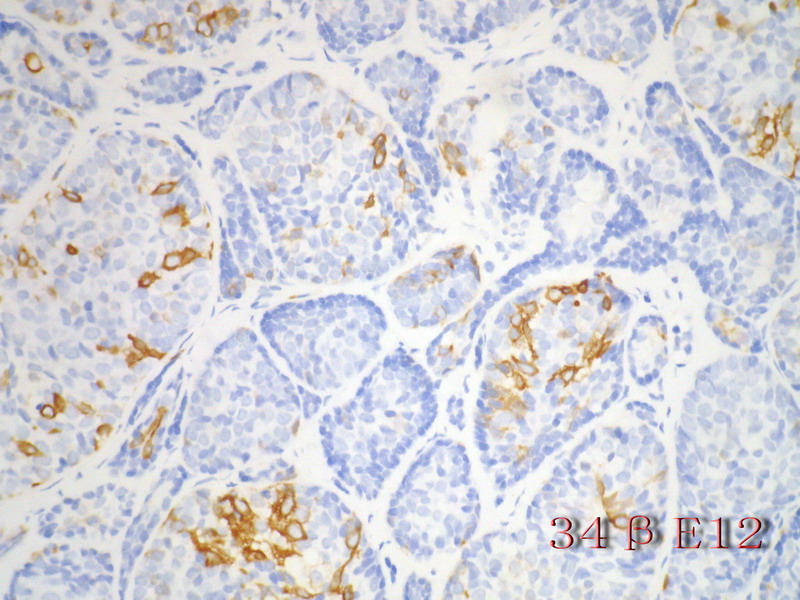
名称:图13
描述:图13
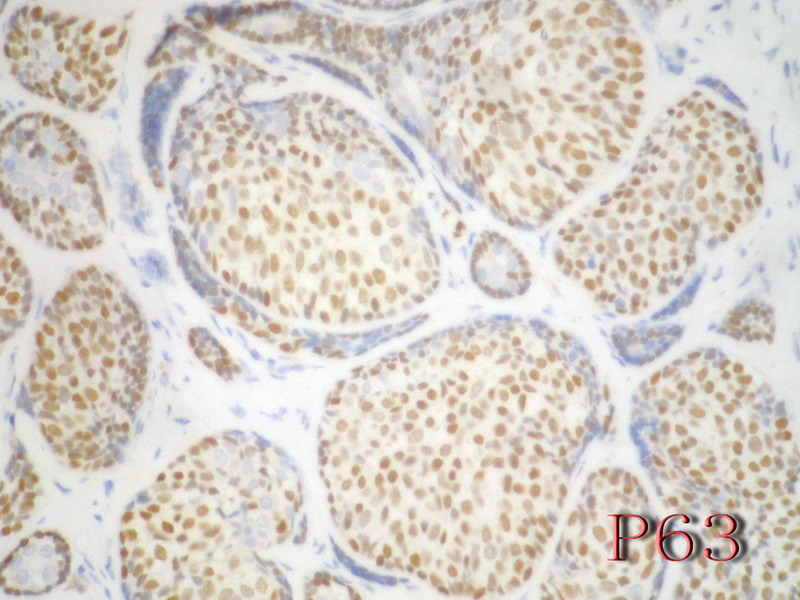
名称:图14
描述:图14
-
本帖最后由 于 2010-09-09 16:26:00 编辑

- 病理,让疾病明明白白。
相关帖子
- • 边界清楚的乳腺包块
- • 右乳肿块,新加免疫组化结果
- • 乳癌类型?
- • 乳腺肿块,请会诊
- • 左侧乳腺外上象限肿物,髓样癌?
- • 乳腺肿块
- • 女,29岁,发现右乳肿块,停止哺乳后手术切除肿块
- • 请求老师会诊,34岁女,乳腺肿物。
- • 乳腺癌类型?
- • 乳腺微创手术活检,诊断?
-
skyliutong 离线
- 帖子:497
- 粉蓝豆:189
- 经验:878
- 注册时间:2009-02-14
- 加关注 | 发消息
乳腺腺样囊性(ACC)的形态与唾液腺ACC的形态是相似的。唾腺ACC组织学类型:小管型,筛孔型,实体性,混合型。IHC标记:一般标记CK7+ CK14+ CK17+ CK19+; S-100+ MUC1+ RB+ CD117+:
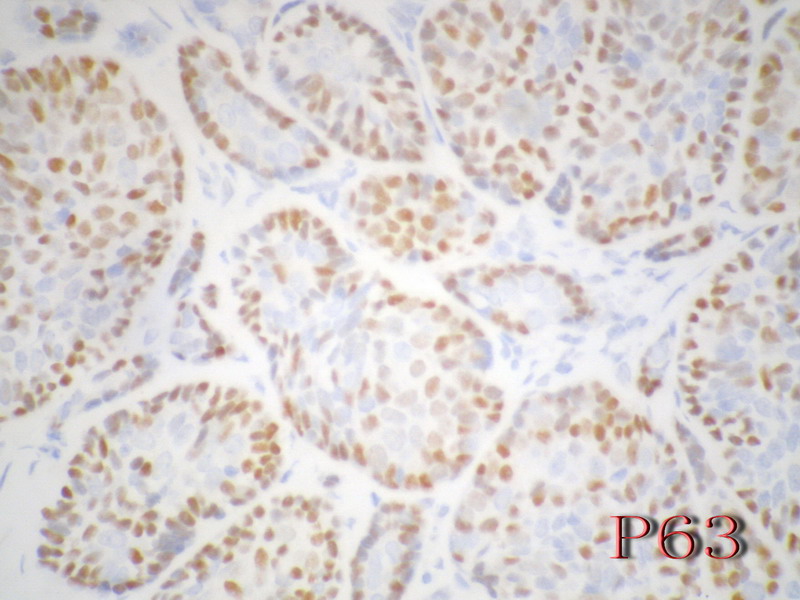
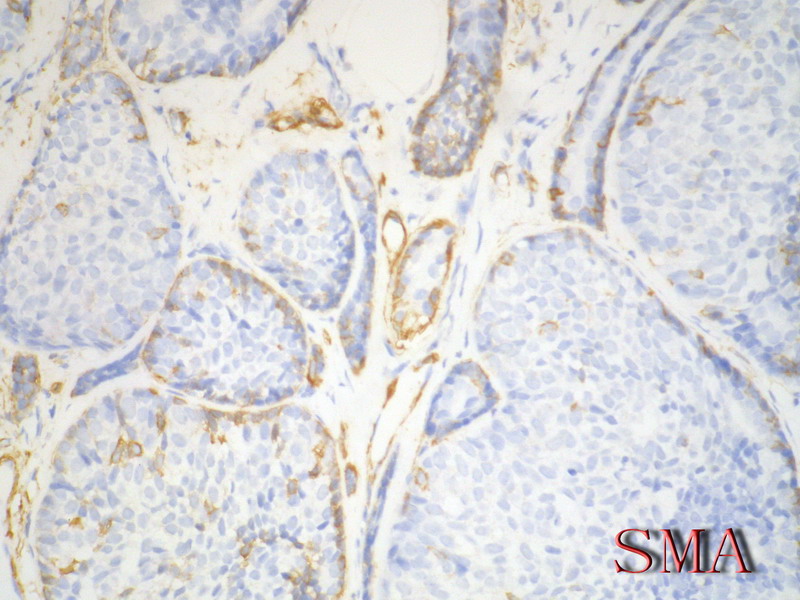
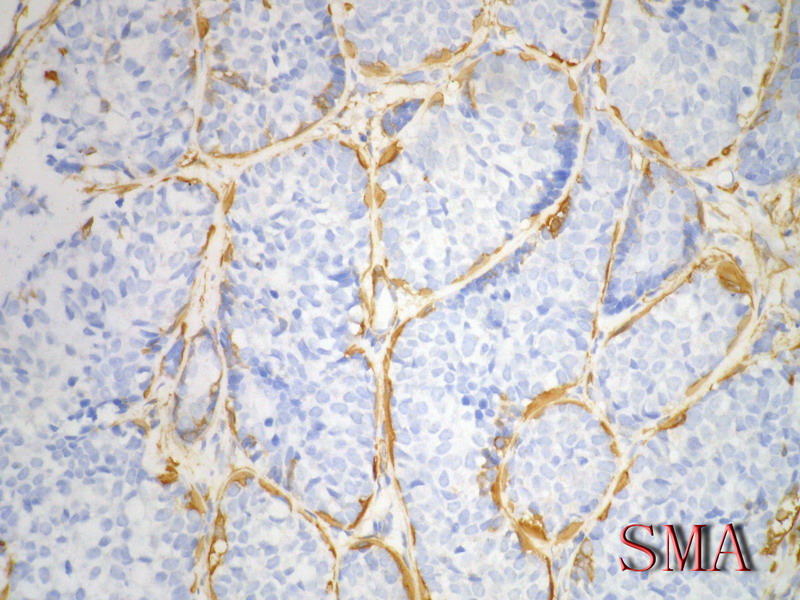
导管上皮细胞:CEA+EMA+;肌上皮分化细胞:CK+ Vimentin+ MSA+ p63+, SMA+ SMMHC+ Calponin+ GFAP 局限+,等。 ER/PR一般为阴性。
注意:乳腺ACC的形态变异比唾腺ACC更多,而且基底细胞样表型时p63-34BE12+。Ro 等提出乳腺ACC可根据肿瘤内实体成分的百分比分为3级:1级 无实体性成分;2级实体性成分小于30%; 3级实体成分大30%。
本例诊断依据:肿瘤浸润性生长,浸润脂肪组织。主要结构为实体细胞团,但可见小管样结构。实体巢周边细胞栅栏样排列,似汗腺圆柱瘤;二种细胞构成(导管上皮和肌上皮),细胞小,比较均一,无核分裂象,无坏死。鉴别1)浸润性小叶癌无二种类型的细胞,肌上皮应该消失,浸润时呈靶形结构和单行排列。2)浸润性导管癌累及小叶时(小叶癌化)肿瘤细胞异型性大,且应有导管癌成分。
以上个人观点仅供参考。

- xljin8
-
本帖最后由 于 2009-11-13 20:42:00 编辑
This is an very interesting case. Thank Dr. XLJin8 's excellent analysis.
My differential dx for this case.
1. lobular lesion, chance is low
2. ACC
3. basoloid carcinoma of breast (BCB)
4. IDC with basal-like phenotype.
Differential dx shoud include all possibilities. Then we will rule out or rule in one by one.
In fact most ACC and BCB are in the category of basal-like carcinoma.
Stains
E-cad
ER, PR, Her2: Most lobular lesions ER/PR+
ACC and BCB: triple negative in most cases
Ckit, P63, SMA positive in ACC, but negative in BCB, high molecular weight CK should be pos in both (see the following abstract, I am not sure).
some basal-like markers ck14, ck5/5, ck17 or EGFR can be positive in both ACC and BCB.
-
本帖最后由 于 2010-09-09 19:00:00 编辑
Ann Diagn Pathol. 2008 Feb;12(1):4-11. Epub 2007 Oct 3.
Basaloid carcinoma of the breast: a review of 9 cases, with delineation of a possible clinicopathologic entity.
Lamovec J, Falconieri G, Salviato T, Pizzolitto S.
Department of Pathology, Institute of Oncology, SI-1000 Ljubljana, Slovenia.
Basaloid carcinoma of the breast (BCB) is an unusual neoplasm composed of basal-type neoplastic cells similar to those found in adenoid cystic carcinoma (ACC), although lacking distinctive features such as a cribriform pattern, a dual neoplastic population (epithelial-myoepithelial/basaloid), and stromal deposits of basement membrane-like material. In this article, we present 9 cases of breast cancer showing overall/predominant basaloid morphology. Patients' ages ranged from 47 to 75 years (mean, 61.4 years). Surgical treatment included mastectomy or quadrant excision with or without axillary dissection. Most tumors had a circumscribed outline and ranged in size from 1.3 to 5.5 cm (mean, 2.5 cm). Microscopically, they featured sheets, nests, and cords of proliferating basaloid tumor cells with ovoid, hyperchromatic nuclei with inconspicuous nucleoli and scant cytoplasm. No foci with characteristics of ACC were found in any of the tumors. Transition into pleomorphic basaloid carcinoma with foci of high-grade ductal carcinoma in situ plus infiltrating ductal carcinoma (IDC) and admixture with grade 3 ductal and sarcomatoid carcinoma was seen in 2 cases. Tumor cells were positive for wide-spectrum keratins and epithelial membrane antigen (9/9) and high-molecular-weight keratins (7/9). They were negative for smooth muscle actin, p63, calponin, and CD10 in all tested cases. Estrogen receptor, progesterone receptor, and HER-2 were negative. Axillary lymph node metastases were seen in 3 cases. At follow-up (range, 10-169 months), 5 patients were alive, 1 with evidence of contralateral breast cancer. Three patients died: one of disseminated BCB metastases, another of liver cirrhosis, and one of disseminated estrogen receptor/progesterone receptor-positive contralateral IDC. One patient was lost to follow-up. We concluded that BCB has some phenotypic and immunohistochemical features enabling its distinction from ACC or IDC. It appears to be a morphological and possibly a clinical entity. Compared with ACC, BCB appears to be more aggressive and may entail a more guarded prognosis.
-
本帖最后由 于 2010-09-09 19:01:00 编辑
Mod Pathol. 2005 Oct;18(10):1277-82.
Immunoreactivity for c-kit and p63 as an adjunct in the diagnosis of adenoid cystic carcinoma of the breast.
Mastropasqua MG, Maiorano E, Pruneri G, Orvieto E, Mazzarol G, Vento AR, Viale G.
Division of Pathology and Laboratory Medicine, European Institute of Oncology and University of Milan, School of Medicine, Milan, Italy.
Adenoid cystic carcinoma of the breast represents a unique clinicopathologic entity with a variable histological appearance and a relatively indolent clinical course in most of the cases. Adenoid cystic carcinoma may be difficult to differentiate from infiltrating duct carcinomas, and in particular from tubular and cribriform carcinomas, especially in core or vacuum-assisted biopsies. We evaluated the prevalence of c-kit, p63, and e-cadherin immunoreactivity in a series of 20 adenoid cystic carcinomas, comparing the results with those obtained in a series of infiltrating tubular carcinomas and infiltrating cribriform carcinomas. The hormone receptor status, proliferation labeling index, and HER/2 immunoreactivity had been previously investigated in all the cases. Three (15%) adenoid cystic carcinomas and all infiltrating tubular and cribriform carcinomas showed estrogen receptor and/or progesterone receptor immunoreactivity (P < 0.00001 for estrogen and P = 0.00002 for progesterone receptors). Adenoid cystic carcinomas consistently lacked any immunoreactivity for HER/2, whereas three (15%) infiltrating and cribriform carcinomas showed weak and incomplete membrane staining (P = 0.23077). Membranous immunoreactivity for c-kit was found in all except one (predominantly basaloid) adenoid cystic carcinomas (95%), and in none of the infiltrating tubular and cribriform carcinomas (P < 0.00001). Nuclear immunoreactivity for p63 was found in all except three (predominantly basaloid) adenoid cystic carcinomas (85%) and in none of the infiltrating tubular and cribriform carcinomas (P < 0.00001). All infiltrating tubular and cribriform carcinomas and 18/20 (90%) adenoid cystic carcinomas showed immunoreactivity for e-cadherin (P = 0.48718). In summary, adenoid cystic carcinomas showed the following phenotype: estrogen receptor-/progesterone receptor-/c-kit+/p63+ (13 cases, 65%), estrogen receptor-/progesterone receptor/c-kit+/p63- (three cases, 15%), estrogen receptor-/progesterone receptor-/c-kit-/p63+ (one case, 5%), estrogen receptor+/progesterone receptor+/c-kit+/p63+ (two cases, 10%), and estrogen receptor+/progesterone receptor-/c-kit+/p63+ (one case). By contrast, all the infiltrating tubular and cribriform carcinomas showed the estrogen receptor+/progesterone receptor+/c-kit-/p63- phenotype. Our data provide evidence that immunoreactivity for c-kit and/or p63 may be useful in differentiating adenoid cystic carcinomas from other types of breast cancer.
-
本帖最后由 于 2010-09-09 19:01:00 编辑
Breast Cancer Res. 2007;9(5):R65.
Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas.
Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ.
Division of Radiation Oncology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands.
Comment in:
INTRODUCTION: Breast cancer is a heterogeneous group of tumors, and can be subdivided on the basis of histopathological features, genetic alterations and gene-expression profiles. One well-defined subtype of breast cancer is characterized by a lack of HER2 gene amplification and estrogen and progesterone receptor expression ('triple-negative tumors'). We examined the histopathological and gene-expression profile of triple-negative tumors to define subgroups with specific characteristics, including risk of developing distant metastases. METHODS: 97 triple-negative tumors were selected from the fresh-frozen tissue bank of the Netherlands Cancer Institute, and gene-expression profiles were generated using 35K oligonucleotide microarrays. In addition, histopathological and immunohistochemical characterization was performed, and the findings were associated to clinical features. RESULTS : All triple-negative tumors were classified as basal-like tumors on the basis of their overall gene-expression profile. Hierarchical cluster analysis revealed five distinct subgroups of triple-negative breast cancers. Multivariable analysis showed that a large amount of lymphocytic infiltrate (HR = 0.30, 95% CI 0.09-0.96) and absence of central fibrosis in the tumors (HR = 0.14, 95% CI 0.03-0.62) were associated with distant metastasis-free survival. CONCLUSION: Triple-negative tumors are synonymous with basal-like tumors, and can be identified by immunohistochemistry. Based on gene-expression profiling, basal-like tumors are still heterogeneous and can be subdivided into at least five distinct subgroups. The development of distant metastasis in basal-like tumors is associated with the presence of central fibrosis and a small amount of lymphocytic infiltrate.
-
本帖最后由 于 2010-09-09 19:02:00 编辑
Radiologia. 2006 Jul-Aug;48(4):235-40.
[Adenoid cystic carcinoma of the breast]
[Article in Spanish]
de Luis E, Apesteguía L, Noguera JJ, Pina L, Martínez-Regueira F, Miguel C, Sáenz J.
Servicio de Radiología, Clínica Universitaria de Navarra, Pamplona, España. edeluis@unav.es
OBJECTIVE: To review the clinical presentation and imaging findings of adenoid cystic carcinoma (ACC). MATERIAL AND METHODS: We performed a retrospective study of the period between January 1990 and July 2004, comprising five cases of ACC of the breast, all in women, among 4,036 malignant lesions diagnosed (0.12%). We reviewed the available imaging studies (mammography in all five cases, ultrasound in four, and magnetic resonance in one). We also reviewed the clinical presentation and evolution in all patients. RESULTS: Three patients presented with palpable lesions. Mammographic findings consisted of irregular, ill-defined nodules in three cases, a well-defined rounded nodule in one, and an asymmetrical density in the other. No microcalcifications were observed in any case. Ultrasound examination showed ill-defined polylobulated nodules in three cases and a well-defined, rounded nodule with small cysts inside in the remaining case that showed intense vascularization in the Doppler study. The only case studied by magnetic resonance was seen as a rounded nodule that showed heterogeneous contrast uptake, well-defined margins, and an enhancement curve considered highly suspicious for malignancy. Treatment was tumorectomy together with radiotherapy in all cases. Four patients remain asymptomatic at present (mean follow-up = 64 months) and one presented lung and liver metastes twelve years after the diagnosis of ACC. CONCLUSION: ACC is an uncommon breast tumor with varied radiologic appearance, although moderately or highly suspicious lesions predominate. We consider the absence of microcalcifications in these tumors to be noteworthy. The prognosis is generally good, although the possibility of remote metastasis exists.
-
本帖最后由 于 2010-09-09 19:02:00 编辑
Mod Pathol. 2006 Oct;19(10):1351-7. Epub 2006 Jun 30.
Immunophenotypic overlap between adenoid cystic carcinoma and collagenous spherulosis of the breast: potential diagnostic pitfalls using myoepithelial markers.
Rabban JT, Swain RS, Zaloudek CJ, Chase DR, Chen YY.
Department of Pathology, University of California, San Francisco, CA 94143, USA. joseph.rabban@ucsf.edu
Adenoid cystic carcinoma of the breast is a rare neoplasm whose cribriform architecture may mimic invasive cribriform carcinoma, cribriform ductal carcinoma in situ, and collagenous spherulosis. The diagnosis may be even more challenging in needle core biopsies. Immunohistochemical expression of p63 and c-kit distinguishes adenoid cystic carcinoma from invasive cribriform carcinoma and ductal carcinoma in situ. A formal comparison of the immunophenotype of adenoid cystic carcinoma to collagenous spherulosis has not been reported. Of concern is the overlap in myoepithelial markers between these two entities. Both may express S100, smooth muscle actin, and p63. This overlap may cause diagnostic confusion yet is under-emphasized in the literature. The expression profile of newer myoepithelial markers has not been studied in this setting. We evaluated smooth muscle actin, p63, calponin, smooth muscle myosin heavy chain, as well as c-kit, in nine cases of cribriform pattern adenoid cystic carcinoma of the breast in comparison to 12 cases of collagenous spherulosis. Both entities strongly expressed p63 and smooth muscle actin; in adenoid cystic carcinoma, the basaloid myoepithelial-like tumor cells expressed these markers, but the ductular epithelial cells did not. Neither calponin nor smooth muscle myosin heavy chain was expressed in adenoid cystic carcinoma but both were strongly expressed in collagenous spherulosis. Whereas the ductular epithelial cells of adenoid cystic carcinoma were positive for c-kit in all cases, collagenous spherulosis was negative for c-kit. Positive p63 expression by a cribriform breast lesion is not sufficiently specific to confirm a diagnosis of adenoid cystic carcinoma. A broader panel that includes calponin or smooth muscle myosin heavy chain and c-kit is required to exclude collagenous spherulosis in settings in which the distinctive morphologic features that separate these entities are not conspicuously present. Reliance on p63 or smooth muscle actin alone poses a potential diagnostic pitfall in evaluating cribriform breast lesions.



















