| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- B22Breast papillary lesion cqz (1)
| 姓 名: | ××× | 性别: | f | 年龄: | 52 |
| 标本名称: | Breast segmental mastectomy | ||||
| 简要病史: | Breast lesion | ||||
| 肉眼检查: | |||||
failed to poste the photos and try again.
Your diagnosis
Differential diagnoses
What immunostains will be useful?
-
本帖最后由 于 2009-02-17 09:36:00 编辑
Key for Figures:
1. 10x smooth muscle myosin heavy chain (SMMHC)
2. 10x SMMHC
3. 10x SMMHC tumor edge
4. 20x SMMHC
5. 10x p63
6. 10x p63
7. 10x p63 tumor edge
Now you have the IHC results. Please write down your dx. Is it the same as the one you though before? This is a challenge case. Very happy to know all friends' dx and discussion. It is better to write your diagnosis and also explain why. As pathologists we make diagnosis for our cases based on the reasons.
I will join the discussion few weeks later,
Thnaks,
cqz
- 文章:1808
- 积分:10455
- 经验:2476
- 注册:2007-6-30 21:1
- 文章:3
- 积分:15
- 经验:3
- 注册:2008-6-25 21:53
- 文章:2487
- 积分:14420
- 经验:3267
- 注册:2007-2-14 22:47
|
| |||||||||||||
|
| ||||||||||
|
| |||||||||||||||

- 没有完美的个人,只有完美的团队
这是导管内乳头状病变。需在“瘤”与“癌”之间鉴别。从HE看,细胞形态一致,核增大,圆形或卵圆形,染色质丰富而细腻,可见1-2个小核仁。乳腺癌癌细胞的特点之一就是“太一致”,易被误为神经内分泌癌。从IHC看,肌上皮细胞在异型上皮细胞巢周围分布,而不是深入乳头轴心内。综上,考虑为导管内乳头状瘤伴重度不典型增生、癌变,或癌在导管内乳头状瘤内。
期待cqzhao老师的讲解!

- If you have great talents, industry will improve them; if you have but moderate abilities, industry will supply their deficiency. 如果你很有天赋,勤勉会使其更加完美;如果你能力一般,勤勉会补足其缺陷。
| 以下是引用Liu_Aijun在2008-10-5 11:06:00的发言:
这是导管内乳头状病变。需在“瘤”与“癌”之间鉴别。从HE看,细胞形态一致,核增大,圆形或卵圆形,染色质丰富而细腻,可见1-2个小核仁。乳腺癌癌细胞的特点之一就是“太一致”,易被误为神经内分泌癌。从IHC看,肌上皮细胞在异型上皮细胞巢周围分布,而不是深入乳头轴心内。综上,考虑为导管内乳头状瘤伴重度不典型增生、癌变,或癌在导管内乳头状瘤内。 期待cqzhao老师的讲解! |
支持刘老师的看法!
请教cqzhao老师:是否需标记ER?在增生性病变,ER表达分布不均匀,而在癌则是弥漫强阳性,不知对否?请赐教。谢谢!

- 广州金域病理
-
stevenshen 离线
- 帖子:343
- 粉蓝豆:2
- 经验:343
- 注册时间:2008-06-03
- 加关注 | 发消息
| 以下是引用笃行者在2008-10-12 20:09:00的发言:
导管内乳头状肿瘤。按照WHO标准,如果达到以下两个标准中任何一项即可诊断导管内乳头状癌: 1、肿瘤90%以上的区域肌上皮消失,无论有无明显上皮增生。或/和: 2、肿瘤90%以上的区域呈现低级别导管内癌图像,无论有无肌上皮存在。 如果达不到以上标准,则可诊断不典型导管内乳头状瘤。 很好的病例,谢谢cqzhao老师!期待您的最后答案。 |
第6楼显示肌上皮存在,下一步应该确定增生的性质:UDH/ADH/DCIS?
形态非常一致,范围也足够大,考虑增生的性质为DCIS。IHC检测ER、CK5、CAM5.2等有助于鉴别。
那么参照第2条标准,如果DCIS范围>90%,考虑导管内乳头状癌。如果<90%为不典型乳头状瘤。
另一种诊断名称“导管内乳头状瘤伴DCIS”可能也比较合适。
非常值得反复学习的好病例,谢谢楼主。期待您的最后诊断和讲解。谢谢!

华夏病理/粉蓝医疗
为基层医院病理科提供全面解决方案,
努力让人人享有便捷准确可靠的病理诊断服务。
-
本帖最后由 于 2008-10-29 18:04:00 编辑
Thank for reviewing this case.
Histologically, the papillary lesion demonstrates papillary-like proliferation with uniform cells. I cannot appreciate the obvious myoepithelial cell distribution within the lesion or surrounding the entire papillary lesion. Some cystic space can be seen in the low power. My impression is that this is a papillary carcinoma with cyst and without myoepithelial linging surrounfing the lesion. So I released the case in the same day and diagnosed as intracyatic papillary caricinoma (IPC) or encapsulated papillary carcinoma (EPC). I still ordered IHC for myoepithelial markers. I was surprised to read the ihc rslides above in the second day. Myoepithelial cells are present surrounding the papaillary lesion (ruled out EPC) and focally within the papillary lesion. The final diagnosis is DCIS involving the papilloma. I revised my diagnosis and noticed the surgen immediately enven though the clinical treatment is the same. The lesson I learn is that I will do IHC for papillary lesion until the cases are classic intraductal papilloma.
The case confused me is that cytologic features of the papillae or glands are exactly same in the entire papillary lesion regardless the areas with or without myoepithelial cells.
If you still think this is a intraductal papilloma after the IHC. Suggest that you read the book chapter of paillary lesion and the interpretation of myoepithelial markers again. A lot of papillary structures loss the myoepthelial cells in this case. It should not be a benign intraductal papilloma.
There are no good standards for diagnosis papillary lesion such as atypical papilloma, DCIS arising from papillaoma, papillary carcinoma. Tavasoli who worked at AFIP for many years before 2002 ( you may read her breast book if you are interested in breast pathology) used 1/3 as cut line. Atypical papilloma: atypical proliferation less than 1/3 of the papillary lesion. DCIS: atypical proliferation >1/3 to 90%; Papillary carcinoma: atypical >90%. Now most people used the criteria: atypical papilloma--focal atypical proliferation like ADH; DCIS arising from papillaoma--focal atypical proliferaiton like DCIS; papillary carcinoma---DCIS almost (or 90%) or entirely involving the papillary lesion. Pathologically or clinically there are no differences between DCIS arising or involving papilloma and papillary carcinoma. Both are types of DCIS. Also you can call small papillary ca as DCIS, papillary pattern.
In the US excional biospy will always be performed if atypical papilloma is diagosed in the breast core biopsy.
I do not have the experience about the usage of ER in the diagnosis of papillary lesion. I think it will not be useful.
Thanks,
cqz
abin译:
谢谢大家参与讨论。
组织学上,乳头状病变呈乳头样增生,细胞一致。我不能识别病变内或整个乳头状病变周围是否存在明显的肌上皮细胞。低倍镜下可见囊性腔隙。我的印象是有囊的乳头状癌,病变周围没有肌上皮衬覆。因此同一天我发了报告,诊断为囊内乳头状癌(IPC)或者有囊包裹的乳头状癌(EPC)。但是我仍然安排做了肌上皮标记。第二天看到免疫组化结果时,我很惊讶。乳头状病变周围存在肌上皮细胞(可排除EPC),乳头状病变内部也局灶存在肌上皮!最后诊断是DCIS累犯乳头状瘤。我修改了诊断并且立即通知外科医生,尽管临床处理相同。我从中学到的教训是:乳头状病变要做免疫组化,直到确信它确实是典型的导管内乳头状瘤。
令我困惑的是,肌上皮存在的区域或无肌上皮的区域,整个乳头状病变中的乳头或腺体内的细胞学特征非常一致。
如果看过免疫组化之后,你仍然认为它是导管内乳头状瘤,建议你再次阅读乳头状病变和肌上皮标记物的有关章节。本例中大部分乳头状结构丢失肌上皮。它不应该是良性的导管内乳头状瘤。
对于不典型乳头状瘤、起源于乳头状瘤内的DCIS、乳头状癌,这些乳头状病变没有形成较好的诊断标准。Tavasoli在2002年以前在AFIP工作过多年,如果你对乳腺病理有兴趣,可能读过她的书。她使用1/3作为分界线。不典型乳头状瘤:不典型增生<1/3乳头状病变;起源于乳头状瘤内的DCIS:不典型增生介于1/3~90%之间;乳头状癌:不典型增生>90%。现在大多数接受以下标准:不典型乳头状瘤:与ADH相似的局灶不典型增生;起源于乳头状瘤内的DCIS:与DCIS相似的局灶不典型增生;乳头状癌:几乎全部(或90%)为DCIS或DCIS完全累犯乳头状病变。
至于DCIS起源于乳头状瘤,还是DCIS累犯乳头状瘤,病理学或临床上无法区分。二者都属于DCIS的不同类型。你也可以把小灶乳头状癌称为DCIS,乳头状型。
在美国,如果粗针穿刺活检诊断了不典型乳头状瘤,通常要进行切除活检。
在乳头状病变中我没有使用ER的经验。我认为这没有帮助。
谢谢,
cqz
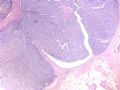
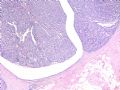
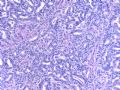
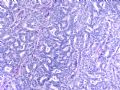
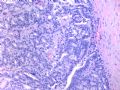


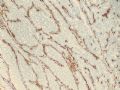







 intraductal papilloma
intraductal papilloma