| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- 2012年第18期——肺部肿物(已点评)
女 10岁, 2周前无明显诱因出现咳嗽咳痰,阵发性连声咳,痰不易咳出,无发热咯血盗汗,无夜间呼吸困难,无胸闷胸痛,当地医院抗感染效果不佳,胸片:左下肺占位,炎性假瘤可能性大,左下肺局限性肺气肿,肺炎。
送左下肺及肿物:12×8.5×
本例图片采用麦克奥迪MoticBA410显微镜+MoticPro285A摄像头采集制作。
点评专家:李向红(43楼 链接:>>点击查看<< )
获奖名单:红胜火(3楼 链接:>>点击查看<< )
-
本帖最后由 草原 于 2012-06-10 20:26:06 编辑
感谢全子老师提供的好病例供大家学习,感谢广大网友的积极参与,非常感谢李向红老师在百忙之中做出的精彩点评!下面我转发李老师的点评:
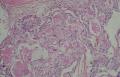
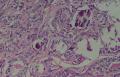
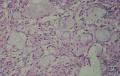
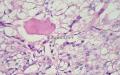
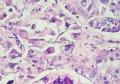
同意原单位和大多数网友的诊断,本例为儿童肺原发的粘液表皮样癌。谈一下个人的诊断思路。
1. 确定原发还是继发 儿童肺的原发肿瘤少于继发肿瘤,但本例肿瘤为孤立性,且体积较大,临床表现也是呼吸道症状,因此首先考虑是原发肿瘤。
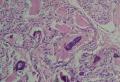
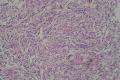
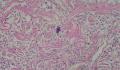
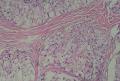
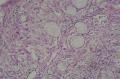
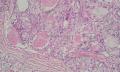
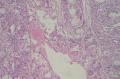
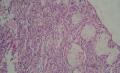
2. 确定良恶性和肿瘤的分型 实际工作中判断肿瘤的良恶性主要还是靠镜下观察,本例镜下组织学形态可以除外经验中最常见的儿童良性肿瘤如:乳头状瘤,错构瘤和炎性假瘤(即炎性肌纤维母细胞瘤)。进一步观察所提供的组织切片,个人感觉图片2和11显示了非常典型的粘液表皮样癌的特点:组织结构为腺管状和实性上皮性肿瘤,伴有明确的粘液小池,上皮性肿瘤细胞又可区分出中间型细胞,粘液分泌细胞和胞浆完全透亮的细胞,而且多数肿瘤细胞核看起来比较善良。但是,图片4、5、6、7、8、16、17、19、和21中有的腺样结构更明显,粘液池中出现钙化,细胞异型性变得明显,还有嗜酸性细胞的实性排列,不是粘液表皮样癌的常见形态,需要更进一步的鉴别诊断。我个人的主要鉴别诊断考虑是否为转移性混合性生殖细胞肿瘤(转移性胚胎性癌?因为是儿童,虽然肺内孤立的大结节不支持转移瘤,但均不是绝对的),需要有免疫组化的支持,因此感谢原单位做了CD30,结果为阴性,除外了这一可能。与其他原发或继发肺内的透明细胞肿瘤如糖瘤或转移性肾透明细胞癌因形态学相差较远(这两个肿瘤都不应该有这么明显的分泌粘液的细胞和粘液池形成)没有在鉴别诊断中考虑。与儿童肺母细胞瘤的形态学(双向分化)差异也较大,因此也未作为主要的鉴别诊断。有网友考虑要与上皮-肌上皮癌鉴别,本例的细胞形态多于两种类型,又有名显的粘液池形成,不支持上皮-肌上皮肿瘤,也可以用免疫组化的方法检测P63的表达, 粘液表皮样癌应是CK表达的细胞同时表达P63而上皮肌上皮癌中的分泌上皮细胞应该不表达P63。此外, 一些级别较高的粘液表皮样癌和分化差的鳞状上皮癌很难区分,此时做粘液染色非常有帮助,前者可以找到散在分布的分泌粘液的细胞。
3. 肿瘤的分级 根据上传的图片,我个人认为是II级(中度恶性),因实性的区域比较多,大的粘液池不是很多,未看到明确的核分裂像和坏死,多数肿瘤细胞的核级也不高,未见广泛的促纤维增生和炎性反应。粘液表皮样癌的分级目前并不是很同意,有三级和二级分法,更客观的分级今后可能还有赖于分子分型。请原单位再补充一下肿瘤是否浸润胸膜,以及有无淋巴结的转移,毕竟目前与预后最为相关的还是TNM分期。
4. 延伸阅读 本例的诊断过程是和大家一起学习的过程,我也快速浏览了一下相关的文献(相信网友们比我读的更全面和仔细),主要的收获是粘液表皮样癌的分级已有分子生物学的方法,更重要的是已有免疫组化的方法来检测MECT1-MAML2融合基因和蛋白,有融合基因的粘表的复发、转移和肿瘤相关的死亡风险要明显低于无融合的患者(P = 0.0012),可使我们的分级变得更加客观,下列文献供分享。
Genes Chromosomes Cancer. 2006 May;45(5):470-81
Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene.
Behboudi A, Enlund F, Winnes M, Andrén Y, Nordkvist A, Leivo I, Flaberg E, Szekely L, Mäkitie A, Grenman R, Mark J, Stenman G.
Lundberg Laboratory for Cancer Research, Department of Pathology, Göteborg University, Sahlgrenska University Hospital, Göteborg, Sweden.
Abstract
Mucoepidermoid carcinomas (MECs) of the salivary and bronchial glands are characterized by a recurrent t(11;19)(q21;p13) translocation resulting in a MECT1-MAML2 fusion in which the CREB-binding domain of the CREB coactivator MECT1 (also known as CRTC1, TORC1 or WAMTP1) is fused to the transactivation domain of the Notch coactivator MAML2. To gain further insights into the molecular pathogenesis of MECs, we cytogenetically and molecularly characterized a series of 29 MECs. A t(11;19) and/or an MECT1-MAML2 fusion was detected in more than 55% of the tumors. Several cases with cryptic rearrangements that resulted in gene fusions were detected. In fusion-negative MECs, the most common aberration was a single or multiple trisomies. Western blot and immunohistochemical studies demonstrated that the MECT1-MAML2 fusion protein was expressed in all MEC-specific cell types. In addition, cotransfection experiments showed that the fusion protein colocalized with CREB in homogeneously distributed nuclear granules. Analyses of potential downstream targets of the fusion revealed differential expression of the cAMP/CREB (FLT1 and NR
5. 获奖网友应该是guo2131220,虽然mmmjjj222的分析同样精彩,但前者是初始诊断者。

- Stop walking today and you'll have to run tomorrow.
-
pathologybz 离线
- 帖子:14
- 粉蓝豆:2
- 经验:20
- 注册时间:2012-02-28
- 加关注 | 发消息
-
本帖最后由 草原 于 2012-06-12 13:27:48 编辑
各位的抗议有效,李老师又看了此例,刚刚回复如下:
不好意思,各位网友,因是第一次参加这样的活动,对版面的熟悉程度不够,错评了优胜者,在此纠正。我想应该是网友红胜火获得此期的优胜奖(希望这次没有再搞错哦)。总之,原则是第一位独立诊断的网友,其实只要是独立正确诊断的网友就都是“潜在”的获奖者,对吧。再次抱歉。谢谢大家的宽容。
代表各位网友再次感谢李老师! 恭喜红胜火网友再次获奖!希望“潜在”的获奖者继续努力!
恭喜红胜火网友再次获奖!希望“潜在”的获奖者继续努力!
- Stop walking today and you'll have to run tomorrow.

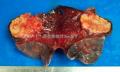
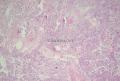
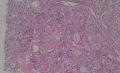
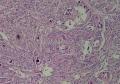













 有些不好意思。
有些不好意思。












