| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- 2012年第12期-乳腺包块(已点评)
- 图1
- 图2
- 图3
- 图4
- 图5
- 图6
- 图7
- 图8
- 图9
- 图10
- 图11
- 图12
- 图13
- 图14
- 图15
- 图16
- 图17
- 图18
- 图19
- 图20
- 图21
- 图22
- 图23
- 图24
- 图25
- 图26
- 图27
- 图28
- 图29
- 图30
- 图31
- 图32
- 图33
- 图34
- 图35
- 图36
- 图37
- 图38
- 图39
- 图40
- 图41
- 图42
| 性别 | 女 | 年龄 | 41岁 | 临床诊断 | 乳腺肿瘤 |
|---|---|---|---|---|---|
| 临床症状 | 右侧乳腺肿块半年余,PE:右侧乳腺外上象限下缘近乳晕处触及一3cm×2.5cm大小的类圆形肿块,质韧、界欠清、活动较差。 | ||||
| 标本名称 | 切除的乳腺肿块 | ||||
| 大体所见 | 类圆形包块1枚,大小约5cm×3.5cm×2cm,切面灰白色,质韧。 | ||||
本例图片采用麦克奥迪MoticBA410显微镜+MoticPro285A摄像头采集制作。
点评专家:美国纽约罗彻斯特大学病理与实验医学部 王曦老师 点击查看
点评链接:点击查看
获奖网友:pathologybz
获奖链接:点击查看
点评专家:王曦(76楼 链接:>>点击查看<< )
获奖名单:pathologybz(1楼 链接:>>点击查看<< )
-
本帖最后由 草原 于 2012-09-24 08:43:38 编辑
王曦老师点评:
感谢各位的宝贵意见!我的诊断是:硬化性腺病/腺病瘤。
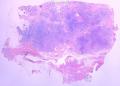
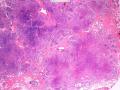
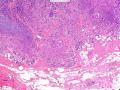
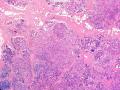
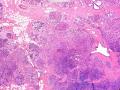
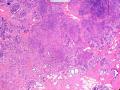
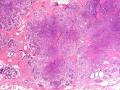
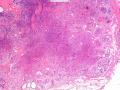
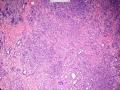
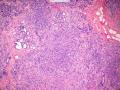
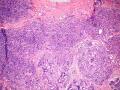
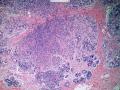
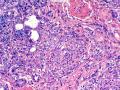
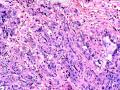
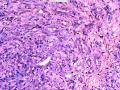
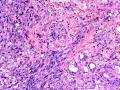
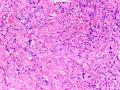
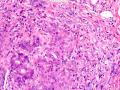
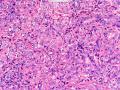
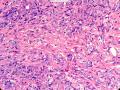
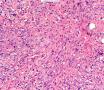
首先,请允许我对腺病/腺病瘤进行一下简单的介绍。腺病,是发生于乳腺终末导管-小叶单位(TDLU)的良性病变。一般见于绝经前女性,平均年龄30岁。临床上,可表现为结节,腺病结节融合时甚至表现为可触及的“肿瘤”,后者更多见于妊娠女性。影像学检查可见伴微钙化的卫星灶(腺病的常见表现),或肿瘤样病变(因此被称为腺病瘤或结节性腺病),从而怀疑浸润性癌。镜下,即使由于腺体/腺泡数量增多而小叶大小显著增大,腺病也会有“小叶结构尚存”这一特征性的低倍表现。腺病的另一低倍特点是带状生长,中央部分受压显著,显得细胞数量多,而周围的腺体/腺泡似乎更加开放或扩张。中倍镜下,腺体/腺泡一般具有规则的、均一的轮廓。高倍镜下,腺体/腺泡一般两层细胞尚存:腔内腺上皮及外围肌上皮。这一特征在腺病的边缘比较容易识别。腔内上皮细胞具有良性导管上皮细胞的特征,比如长椭圆形、重叠,核膜皱褶及核沟,可见核仁,染色质颗粒状,核膜厚等,当然DCIS或LCIS累及腺病时则没有这些特征了。腺上皮周围是显著的肌上皮细胞及基底膜。间质可为透明样胶原排列呈相互吻合的致密条带状。但一般没有粘液样变。有时由于硬化性腺病中的硬化性间质挤压,腺泡结构可不明显,尤其在病灶中央处。此时可行免疫组化标记肌上皮。多个腺病结节相互融合时,可称之为腺病瘤或结节性腺病。一般为2cm左右,但6cm者也有过报道。
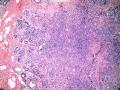
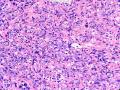
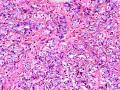
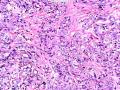
该例,“肿物”5cm。不过,低倍镜下仍可看出其模糊的结节状生长。似乎是结节相互融合,因此形成一个“肿瘤”。带状分布尽管不是很明显,每个结节的大部分区域仍可看出。腺细胞良性表现,具有小的重叠的核,长圆形,核膜厚而折叠,核仁小而显著。腺上皮周边的肌上皮较明显。即使没有免疫组化,根据所提供的图应该也可以很容易的识别。胶原束见于腺体周边,相互吻合。
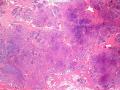
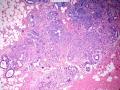
就像很多网友指出的一样,该病变表现为“浸润性生长”的肿物时,最关键的鉴别诊断是浸润性导管癌或小叶癌,尤其在高倍镜下观察病变中央部分时更是如此。当然,我们都知道浸润性癌不会有带状分布的小叶中心性形态,细胞具有恶性特征,浸润性癌不会有肌上皮,一般伴有DCIS。另一个有帮助的细节是浸润性癌的腺体会浸润至脂肪组织,形成无肌上皮的细胞巢或腺体巢团,而腺病的腺体一般仅至纤维组织的边缘。有时脂肪组织中可以见到一些良性腺体,但一般不是无肌上皮的,周围一般会有肌上皮及基底膜(这一点不同于微腺性腺病)。见图1及图2。腺病中另一比较麻烦、但不常见的特征是神经周围侵犯,如果存在这一现象,则容易与浸润性癌混淆。再次声明,免疫组化肌上皮染色是解决这一问题的关键。
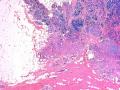
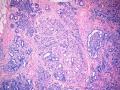
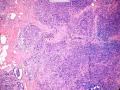
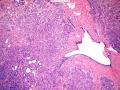
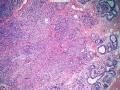
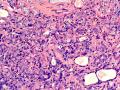
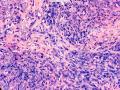
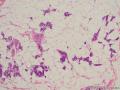
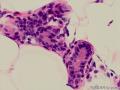
鉴别诊断之二是DCIS累及腺病,很多网友也指出了这一点。仔细观察腺上皮是否具有恶性特征,此外还要观察周围的乳腺组织。一般在周围可见DCIS。当然,也应该做肌上皮免疫标记。见图3及图4。
参考书中提到的其他鉴别诊断还有管状癌,这一例中涉及不到。
几点心得:
1) 低倍镜下,首先观察腺病的小叶中心性和带状分布;
2) 在腺病的边缘过渡区观察细胞的层次,而不要仅在高倍下关注中央区;
3) 观察周围乳腺组织。
在我看来,网友“pathologybz”应该是获奖者。还有几个网友也回答的非常好,但网友“pathologybz”位居第一。

- 赚点散碎银子养家,乐呵呵的穿衣吃饭
Thanks everyone for the opinion! My diagnosis will be: Sclerosing adenosis/adenosis tumor.
Firstly, let me give a general description for adenosis/adenosis tumor. Adenosis is a benign breast lesion arising in terminal ductal lobular unit (TDLU). It is usually found in pre-menopausal women with the mean age 30 years old. Clinically it can present as nodules or even palpable “tumor,” when the adenosis nodules become confluent, which could be more common in pregnant women. Radiation imaging could show stellate area with microcalcification, which is a common feature of adenosis, or mass-like lesion (so-called adenosis tumor or nodular adenosis), hence raising the suspicion for invasive carcinoma. Microscopically adenosis has a characteristic low power view of lobular architecture even though the size of the lobules could be much enlarged due to the multiplication of glands/acini. Another low power feature of adenosis is the zonal growth pattern, with the central part more compressed and appearing more cellular, while the peripheral glands/acini appear more open or dilated. Under medium power view, the glands/acini are generally with regular and uniform contours. Under high power view, the glands/acini always maintain two cell layers: luminal epithelium and myoepithelium. This feature could be better viewed at the intermediate or outermost position of the adenosis. The luminal epithelial cells should have the same features as benign ductal luminal cells, such as long-oval shape, overlapping, nuclear membrane folding and grooving, with nucleoli, granular chromatin, and thick nuclear membrane etc., unless the adenosis is involved by DCIS or LCIS. Surrounding the luminal epithelium are the prominent myoepithelial cells and concentric basement membrane. The stroma could show dense inter-anastomosing bands of hyalinized collagen, such as in sclerosing adenosis. But it is usually not myxoid. Sometimes, the layers of the acinar/glands may not be that obvious because of the distortion or compression by the sclerotic stroma in sclerosing adenosis, especially in the center part. Immunohistochemical stain for myoepithelial markers can be used to clarify this situation. When many adenosis nodules become confluent and merged with each other, the term adenosis tumor or nodular adenosis could be used. It is usually around 2.0 cm, but the “tumors” as large as 6.0 cm have been reported.
The current case presented as 5.0 cm “mass”. However, one can still appreciate a vague nodular growth pattern under low power view. It appears that the nodules become confluent and abutting each other, therefore forming a “mass”. The zonal distribution, even though not that obvious, can still be appreciated in most of the individual nodules. The luminal cells are benign looking, with small overlapping nuclei, long oval shape, folded thick nuclear membrane, and small but distinct nucleoli. Myoepithelial cells are generally prominent surrounding the luminal cells. One can readily appreciate this in the photos provided, even without immunohistochemical stain. The collagen bundles are winding around the acinar/glands, anastomosing with each other.
The most critical differential diagnosis, as pointed out by many friends, is the invasive ductal or lobular carcinoma, when the lesion presented as a mass with an “invasive” growth pattern, especially when one is focused in the center part of the lesion under high power view. As we all know, invasive carcinomas will not maintain a lobular centric morphology with zonal distribution, the cells have all the malignant features, will not have myoepithelial lining, and usually will have DCIS associated with it. One other helpful hint is that the glands of invasive carcinoma will infiltrate into the fatty tissue as naked glands/cell nests, while the glands/acini of adenosis will usually stop at the edge of the fibrous tissue. Sometimes we could see some benign glands in the adipose tissue. But they are certainly not “naked”. They will be always surrounded by myoepithelial cells and basement membrane (other than microglandular adenosis). See fig. 1 and 2. Another notoriously confusing, but not that common feature of adenosis is the perineural invasion which, when present, will be confused with invasive carcinoma. Again, immunohistochemical stain for myoepithelium will be the key to solve the problem.
The second differential diagnosis on the list will be adenosis involved by DCIS, as pointed out by many friends. Other than carefully evaluating the luminal cells to see if they are cancerous, one could also look around in the adjacent breast tissue. You will usually identify the original DCIS in the nearby areas. Of course, one could perform IHC stain for myoepithelium markers too. See fig. 3 and 4.
Other differential diagnosis mentioned in the reference books is tubular carcinoma, which will not be the issue here.
The take home messages are:
1) Take the low power view first to appreciate the lobular centric shape and zonal distribution of adenosis
2) Evaluate the layers and cells at the intermediate to outermost zone of the adenosis, not to focus in the center part under high power view.
3) Look around
To my opinion, I think "pathologybz" could be the one to win the prize. There are a few other friends did equally well as him/her, but he/she is the first to post the answer.
-
xiaoyu_cmu 离线
- 帖子:6
- 粉蓝豆:1
- 经验:41
- 注册时间:2009-06-22
- 加关注 | 发消息