| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- (推荐)Askin 瘤的起源及本质
-
本帖最后由 于 2006-11-06 23:26:00 编辑
挑点刺,不知道是否介意?
文章的确不错,是好文章!
范钦和 Philip W Allen 徐天蓉 周青 张智弘 郑肇巽 - 临床与实验病理学杂志, 2000 Vol.16 No.1 P.8-10
http://www.wanfangdata.com.cn/qikan/periodical.Articles/lcysyblxzz/lcys2000/0001/000103.htm
仔细对照了一下,就是这篇文章,确信自己没有张冠李戴。
挑刺之一:这篇文章是6年前的文章,现在推荐有什么特别的意义?
挑刺之二:这篇文章的作者有多位,而在转述的时候,变成了一位,我还以为是范教授最近的大作
挑刺之三:这篇文章从参考文献来看,都是1996及其以前的文章,现在算起来,文章的内容应该是10年之前的,这10年中,国外对于Ewings family tumour有何进展?这篇文章是否能够代表现在是最新的内容?
只是有一点好奇,现在学习这篇文章有什么意义,顺便挑一点刺,希望不要介意

- 达亦不足贵,穷亦不足悲
-
本帖最后由 于 2006-11-19 14:42:00 编辑
| 以下是引用tumor 在2006-11-19 12:26:00的发言: 挑刺有道理,但也别忘了光挑刺了啊,找点新文献出来让大家学习学习啊 |
既然tumour同志将军了,我也就试着学习一下askin瘤,看能不能有点新的东西。
范教授的文章不错,我们就沿着范教授的文章来继续学习,学习方向,一是了解askin tumour的过去,一则是了解其现在。
在众多的参考书中,谈到了askin tumour ,说是一种具有独特临床特征的肿瘤,然而,并没有谈到这种肿瘤有何独特,所以要了解这个问题,就必须看看范教授提供的第一篇参考文献。
Cancer. 1979 Jun;43(6):2438-51.
Malignant small cell tumor of the thoracopulmonary region in childhood: a distinctive clinicopathologic entity of uncertain histogenesis.
Askin FB, Rosai J, Sibley RK, Dehner LP, McAlister WH.
This report describes a unique clinicopathologic entity characterized as a malignant small cell tumor of the thoracopulmonary region in 20 children and adolescents (average age 14.5 years). There was a female predilection (75%) for this tumor which appeared to originate in the soft tissues of the chest wall or the peripheral lung. The neoplasm tended to recur locally and did not seem to disseminate as widely as some of the other small cell tumors of childhood (rhabdomyosarcoma, Ewing's sarcoma, neuroblastoma and malignant lymphoma). However, the median survival was only 8 months. Electron microscopy of 3 cases suggested a neuroepithelial derivation, but, at the present, the histogeneis remains a subject for further investigation.
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=222426&dopt=Abstract
看看当年的描述,应该对于什么是askin瘤具有一个初步的认识了吧,剩下的就是看看这种肿瘤的研究过程了

- 达亦不足贵,穷亦不足悲
-
本帖最后由 于 2006-11-19 16:18:00 编辑
-
A retrospective review of primary chest wall malignant tumors of childhood collected at the Children's Memorial Hospital of Chicago was undertaken. Among twelve instances of poorly differentiated neoplasms whose uniform, monotonous structure made accurate classification difficult or impossible by conventional histologic study, there were three tumors with features suggestive of neuroectodermal differentiation. Electron microscopic and immunohistologic findings further strengthened this interpretation, despite the fact that none of the patients had evidence of a primary neuroblastoma outside the chest wall. These results and a review of the pertinent literature support the conclusion that neuroectodermal neoplasms in childhood may present in peripheral somatic tissues with greater frequency than is commonly assumed. The importance of this distinction is discussed, particularly the need to distinguish these neoplasms from Ewing's sarcoma.
PMID: 6093982 [PubMed - indexed for MEDLINE]
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=6093982&query_hl=2&itool=pubmed_docsumAm J Surg Pathol. 1986 Feb;10(2):124-33.
Evidence for neural origin and PAS-positive variants of the malignant small cell tumor of thoracopulmonary region ("Askin tumor").
The differential diagnoses of childhood and adolescent tumors composed of small round cells include a distinctive clinicopathological entity called malignant small cell tumor (MSCT) of the thoracopulmonary region in childhood. In the present study, 15 such tumors that fulfilled the criteria by Askin et al. were examined for features of possible neural differentiation by light and electron microscopy (EM). With hematoxylin-eosin stain (H&E) the tumors were made up of small undifferentiated cells; rosette formation was noticed in four cases. By immunohistochemistry all 15 tumors were positive for neuron/specific enolase (NSE), which is a specific marker for neural elements and their tumors including neuroblastomas. Ten of 15 MSCT had positive PAS staining. Ultrastructurally dense core (neurosecretory) granules and cell processes indicative of neuronal differentiation could be recognized in 10 of 14 tumors. The dense core granules were often atypical. Filamentous cytoskeleton, never observed in Ewing's sarcoma, was often present. Based on the current results, MSCT of the thoracopulmonary region can be considered a peripheral neuroectodermal tumor with the possible origin in intercostal nerves. MSCTs are generally misdiagnosed as Ewing's sarcoma due to their primitive appearance in H&E sections and their periodic acid-Schiff positivity. NSE immunostaining, preferably augmented by electron microscopy, is necessary for their correct diagnosis.
PMID: 3953935 [PubMed - indexed for MEDLINE]
对于这两篇文摘的解读我想稍后进行,现在有点其它的事情,告一段落
不知道大家注意到没有,Askin最早称呼这种肿瘤是Malignant small cell tumor of the thoracopulmonary region 这种肿瘤最终以他的名字命名并不是他自己提出来的,而是他人将这种肿瘤冠以他的名字,这就是范教授引用文献2和3
1: Cancer. 1984 Dec 1;54(11):2519-27.
Peripheral neuroectodermal tumors of the chest wall in childhood.

- 达亦不足贵,穷亦不足悲
-
本帖最后由 于 2006-11-19 16:26:00 编辑
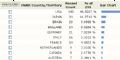
对于范教授的第一篇参考文献进行了一点分析,同时也对askin tumor进行了一点分析,分析图表见上面的图片,这里顺便说一点问题,现在论坛的速度不是太快,上传图片花了我不少时间,第二是我没有办法对于每张图片进行逐一说明,至好下面说明了。
前六张图片是对于引用Askin的大作的文章的分析
第一张,这片文章一共被引用了299次,这当然不包括中国学者的引用,主要是SCI引用,引用的高峰出现在1985-2002年,顶峰是1993年
第二张 引用这片文章的最多国家是美国,几乎占了一半
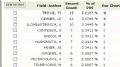
第三张 引用这片文章最多的头十位学者
第四张 引用这片文章最多的10个机构
第五张 引用这片文章最多的领域,可以看出,绝大多数是病理方面的
第六张 引用这片文章最多的杂志
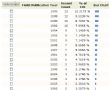
从第7至10张图片说明的是关于askin tumor方面的文章
第七张 发表askin tumor方面文章最多的作者,前十名
第八张 发表askin tumor方面文章最多的国家和地区,美国依然独占鳌头
第九张 研究askin tumor最多的机构
第十张 发表askin tumor的年代,不难看出,最为集中的年代是1992-1995,其次是1998-2001,而从2003年开始,这方面的文章开始减少(为什么)

- 达亦不足贵,穷亦不足悲
-
本帖最后由 于 2006-11-19 16:46:00 编辑
在分析完以上信息的时候,我发现一个有趣的东西,最早称呼askin tumor的并不是范教授引用的那两篇文献而是另有文献
LINNOILA RI, TSOKOS M, TRICHE TJ, et al.
EVIDENCE FOR NEURAL ORIGIN AND PERIODIC ACID SCHIFF-POSITIVE VARIANTS OF THE MALIGNANT SMALL CELL TUMOR OF THORACOPULMONARY REGION (ASKIN TUMOR)
LABORATORY INVESTIGATION 48 (1): A51-A51 1983
FINK IJ, KURTZ DW, CAZENAVE L, et al.
MALIGNANT THORACOPULMONARY SMALL-CELL (ASKIN) TUMOR
AMERICAN JOURNAL OF ROENTGENOLOGY 145 (3): 517-520 1985
时间上要更早一点:D

- 达亦不足贵,穷亦不足悲
由第10张图,我们不难发现,1992年是askin tumor研究的一个分水岭,这一年有什么进展让askin tumor的研究一下之热了一把?在此之前有些什么样的研究?
1992年以前的研究

- 达亦不足贵,穷亦不足悲
-
本帖最后由 于 2006-11-19 19:31:00 编辑
1992年发生了什么?
我们需要看看范教授的第四篇参考文献
Contesso, G., A. Llombartbosch, et al. (1992). "Does Malignant Small Round Cell Tumor of the Thoracopulmonary Region (Askin Tumor) Constitute a Clinicopathological Entity - an Analysis of 30 Cases with Immunohistochemical and Electron-Microscopic Support Treated at the Institute-Gustave-Roussy." Cancer 69(4): 1012-1020.
The morphology and clinical outcome of 30 patients with malignant small round cell tumors located in the thoracopulmonary region (Askin tumor) are reported. Histologically, all tumors had similar patterns, with small round-to-oval cells and a lobulated stroma. Immunohistochemical analysis always resulted in positive staining for one or several neural markers. No significant differences were found compared with the immunomarkers in 26 typical Ewing's sarcomas located outside the thoracic wall. In three specimens, electron microscopy confirmed the presence of membrane-bound neurosecretory granules. It was confirmed that there is a remarkable similarity among all malignant small round cell tumors, including Askin tumor and Ewing's sarcoma. Overall survival was poor with a 2-year rate of 38% and a 6-year rate of 14%.

- 达亦不足贵,穷亦不足悲
努力的试图区分PNET和骨外EWS

- 达亦不足贵,穷亦不足悲
1995年的归纳
到此是不是有一点清楚了?

- 达亦不足贵,穷亦不足悲

- 达亦不足贵,穷亦不足悲

- 达亦不足贵,穷亦不足悲
2001年who,将三者合并,变成了EWS/pnet。具体内容参阅WHO(2001)分类
Soft tissue tumor(第三版)也作了同样的归类。好像也是2001年出版的

- 达亦不足贵,穷亦不足悲




 哈哈! 明白了.
哈哈! 明白了.