| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- B2669女/54岁 左乳腺肿块半年 诊断?
| 姓 名: | ××× | 性别: | 年龄: | ||
| 标本名称: | 左乳腺肿块扩大切除 | ||||
| 简要病史: | |||||
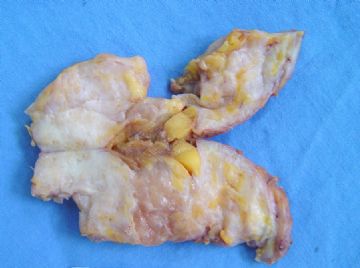
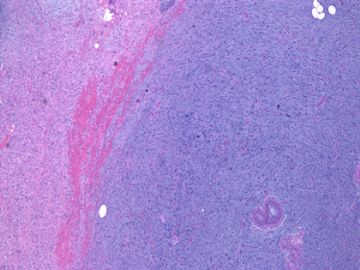
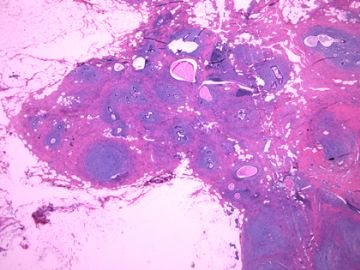
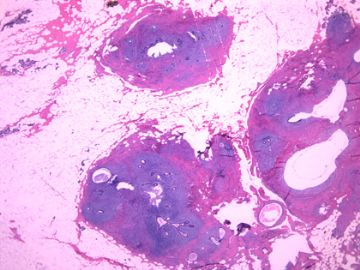
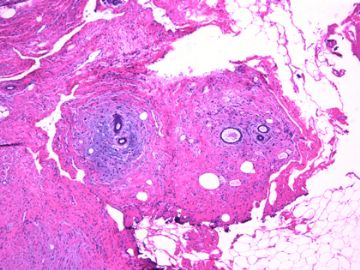
| 肉眼检查: | 组织一块,13.5x11x3cm,切面见3处病灶,直径分别为0.6cm 1.5cm、3cm。灰红-灰白色,部分粘液样,大肿块边界较清楚。 | ||||
-
本帖最后由 于 2010-04-23 12:23:00 编辑

- xljin8
相关帖子
-
qingfengxn 离线
- 帖子:10
- 粉蓝豆:1
- 经验:10
- 注册时间:2010-06-02
- 加关注 | 发消息
| 以下是引用cqzhao在2010-4-28 11:19:00的发言: Most periductal stromal tumors are positive for CD34 based on above studies. Do some ones know phyllodes are negative for CD34? I have no personal experience about CD34 on phyllodes. If it is true CD34 can help to distinguish periductal stromal tumor from classic phyllodes. |
按照赵老师的意思,导管周间质肿瘤表达CD34肯定是阳性的,如果有人知道分叶状肿瘤CD34是阴性表达的话,那标记CD34将有助于区分导管周间质肿瘤和分叶状肿瘤。

- 王军臣
-
本帖最后由 于 2010-06-25 21:41:00 编辑
非常好、非常难得的病例!谢谢金教授拿出来分享!试着谈一下我的理解:
我想这无疑是一个纤维上皮性肿瘤。
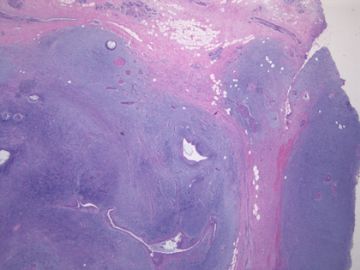
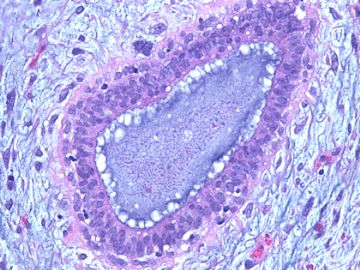
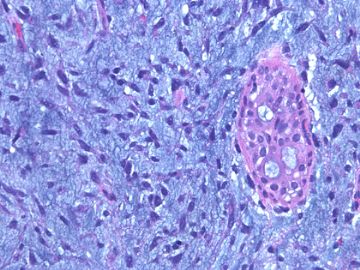
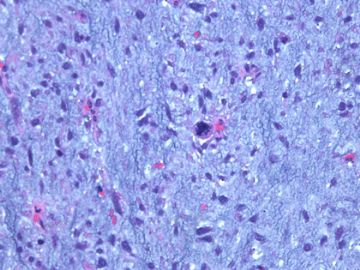
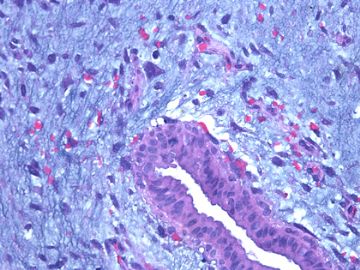
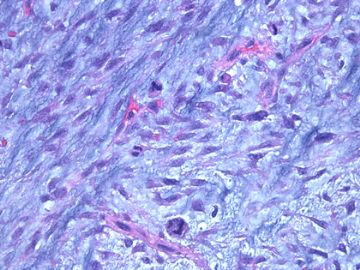
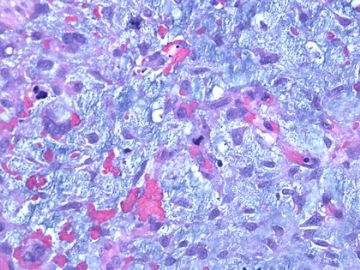
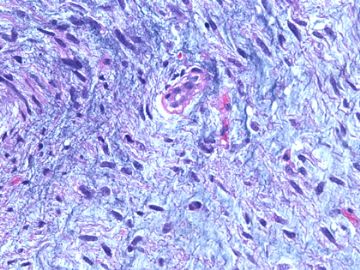
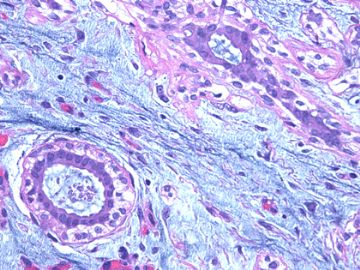
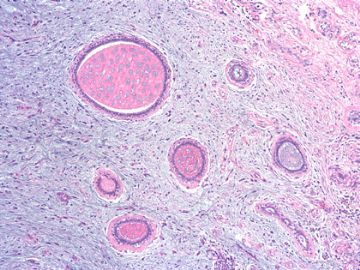
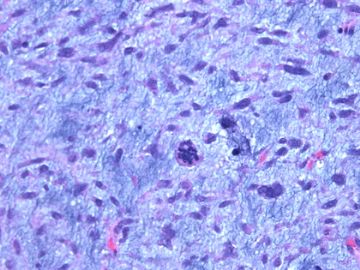
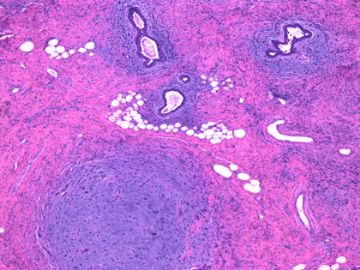
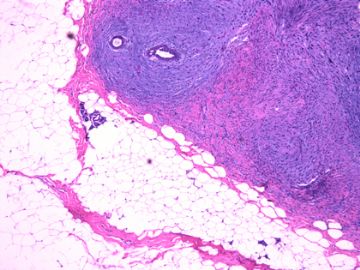
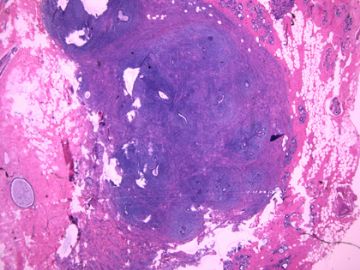
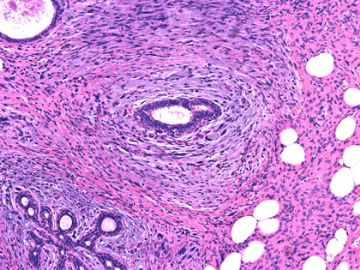
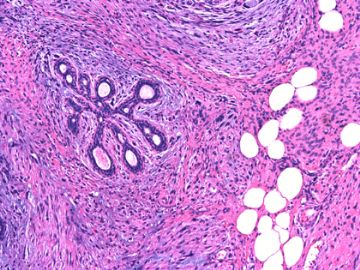
前几幅图(应该是肉眼的较大肿瘤)边界呈浸润性生长、间质过度增生、核分裂较多、有病理性核分裂像,可以看到多个大小不等、不规则的导管结构------应该是一个恶性叶状肿瘤。
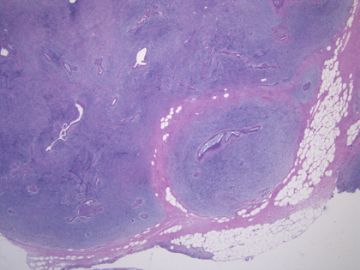
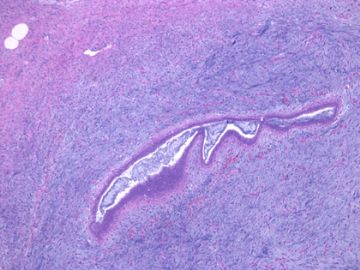
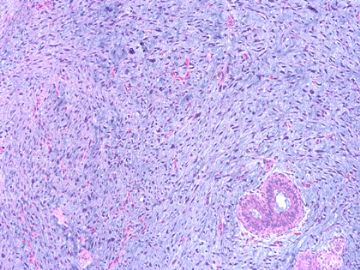
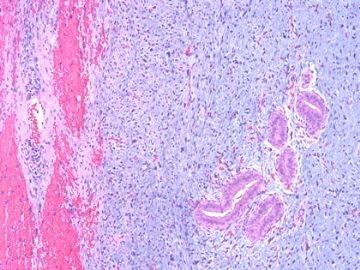
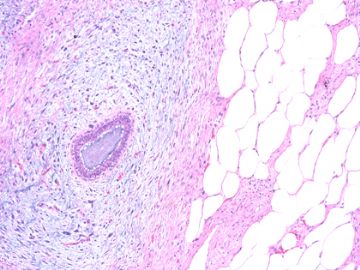
后几幅图(后面8副图)很有特点:许多增生的梭形细胞围绕导管周围排列,导管腔保持开放并还有轻度扩张,一个或几个导管和其周围围绕的梭形细胞形成一个单元、一个结节,其间有多少不等的脂肪组织将他们分隔------这就是所谓的、低级别导管周围间质肉瘤、的典型形态。这种结构如果继续发展、间质细胞过度增生,使每个“结节”相互融合,可能就是上面的几幅图的形态结构了,也就只好诊断“恶性/交界性叶状肿瘤”了。
这可能就是低级别导管周围间质肉瘤与叶状肿瘤之间的关系。
也可能是WHO将两者放在一起、视为同义词的原因吧

- 博学之,审问之,慎思之,明辨之,笃行之。
-
本帖最后由 于 2010-04-27 12:07:00 编辑
| 以下是引用笃行者在2010-4-25 15:30:00的发言:
非常好、非常难得的病例!谢谢金教授拿出来分享!试着谈一下我的理解: 我想这无疑是一个纤维上皮性肿瘤。 前几幅图(应该是肉眼的较大肿瘤)边界呈浸润性生长、间质过度增生、核分裂较多、有病理性核分裂像,可以看到多个大小不等、不规则的导管结构------应该是一个恶性叶状肿瘤。 后几幅图(后面8副图)很有特点:许多增生的梭形细胞围绕导管周围排列,导管腔保持开放并还有轻度扩张,一个或几个导管和其周围围绕的梭形细胞形成一个单元、一个结节,其间有多少不等的脂肪组织将他们分隔------这就是所谓的、低级别导管周围间质肉瘤、的典型形态。这种结构如果继续发展、间质细胞过度增生,使每个“结节”相互融合,可能就是上面的几幅图的形态结构了,也就只好诊断“恶性/交界性叶状肿瘤”了。 这可能就是低级别导管周围间质肉瘤与叶状肿瘤之间的关系。 也可能是WHO将两者放在一起、视为同义词的原因吧 也可能是金教授此贴想告诉我们的吧 |
Borderline fibroepithelial tumor (stromal overgrowth with atypia and increased mitotic figures, yet reactive epithelium)
Good analysis above.
some people think 导管周围间质肉瘤and 叶状肿瘤 sre just termnology issue. If you want to call it as 叶状肿瘤 at least it is bordeline tumor.
It is true this case showes some differences from classic 叶状肿瘤, it is very similar to gynecologic tumor-low grade adenosarcoma with stromal over growth.
The diagnosis of Low-grade periductal stromal sarcoma of the breast with myxoid features is reasonable Maybe we can think Low-grade periductal stromal sarcoma is a special type phyllodes.
Find some papers for some ones who are interested.
r
Pathol Int. 2009 Aug;59(8):588-91.
Low-grade periductal stromal sarcoma of the breast with myxoid features: Immunohistochemistry.
Tomas D, Janković D, Marusić Z, Franceschi A, Mijić A, Kruslin B.
Departments of Pathology, Sestre Milosrdnice University Hospital, Vinogradska 29, Zagreb, Croatia. dtomas@kbsm.hr
Abstract
A 52-year-old woman was admitted with a painful right breast tumor measuring more than 20 cm in largest diameter, which ulcerated the overlying skin. The lesion had appeared 4 years previously but the patient hesitated to seek medical care due to 'fear of cancer'. Microscopically, the tumor was composed of spindle cells that formed cuffs around multiple open tubules and ducts set in an abundant, myxoid stroma. The spindle cells had significant atypia with nuclear pleomorphism, occasional cytoplasmic vacuolation and moderate mitotic activity. The ducts and lobules surrounded by the proliferating tumor cells had minimal distortion, with a pericanalicular growth pattern devoid of the phyllodes pattern. The tumor had a multinodular growth pattern with coalesced and individual tumor nodules, the latter being found mostly at the periphery of the lesion. On immunohistochemistry the tumor cells were positive for smooth muscle actin, CD34, and vimentin, and focally positive for CD10. A diagnosis of low-grade periductal stromal sarcoma (PDSS) with myxoid features was established. PDSS is a distinct low-grade breast sarcoma, the appropriate diagnosis of which requires extensive tumor sampling and additional broad immunohistochemistry. PDSS should not be confused with other spindle cell breast tumors because they require different treatment.
Histopathology. 2008 Jan;52(1):45-57.
Recent developments in the histological diagnosis of spindle cell carcinoma, fibromatosis and phyllodes tumour of the breast.
Histopathology Department, Nottingham University Hospitals, City Hospital Campus, Nottingham, UK. andrew.lee@nuh.nhs.uk
Abstract
This article reviews recent advances in the diagnosis of these three unusual tumours of the breast. Spindle cell carcinoma needs to be considered in the differential diagnosis of many mammary spindle cell lesions: it is important to be aware of the wide range of appearances, including the recently described fibromatosis-like variant. Immunohistochemistry using a broad panel of cytokeratin antibodies is needed to exclude spindle cell carcinoma; there is frequent expression of basal cytokeratins and p63. CD34 is often expressed by the stroma of phyllodes tumours, but does not appear to be expressed by spindle cell carcinoma or fibromatosis. Nuclear beta-catenin is found in about 80% of fibromatoses, but can also be seen in spindle cell carcinomas and phyllodes tumours. Two recent studies have described features useful in the distinction of phyllodes tumour and fibroadenoma on core biopsy, including increased cellularity, mitoses and overgrowth of the stroma, adipose tissue in the stroma and fragmentation of the biopsy specimen. Periductal stromal tumour is a recently described biphasic tumour composed of spindle cells around open tubules or ducts (but no leaf-like architecture) with frequent CD34 expression. The overlap of morphology with phyllodes tumour suggests that it may be best regarded as a variant of phyllodes tumour.
Am J Surg Pathol. 2003 Mar;27(3):343-8.
Periductal stromal tumor: a rare lesion with low-grade sarcomatous behavior.
Department of Breast and Gynecologic Pathology, Armed Forces Institute of Pathology, Washington, DC, USA. amburga@ehmc.com
Abstract
Biphasic breast tumors with benign ductal elements and a sarcomatous stroma lacking a phyllodes architecture are a source of diagnostic problems, particularly because of the lack of an appropriate designation. At the Armed Forces Institute of Pathology, we have used the term "periductal stromal sarcoma" to distinguish these from phyllodes tumors. All cases coded as periductal stromal sarcoma or PDSH were retrieved from the files of the Armed Forces Institute of Pathology. Cases that fulfilled the following criteria were included in this study. The histologic features of periductal stromal sarcoma were defined as 1) a predominantly spindle cell stromal proliferation of variable cellularity and atypia around open tubules and ducts devoid of a phyllodes pattern, 2) one or more often multiple nodules separated by adipose tissue, 3) stromal mitotic activity of >/=3/10 high power fields, and 4) stromal infiltration into surrounding breast tissue. Criteria for periductal stromal hyperplasia included 1) nodular, bland stroma growing as cuffs around normal or altered ducts, 2) no to minimal atypia, and 3) at most 0-2 stromal mitotic figures per 10 high power fields. Immunohistochemistry was used to further characterize these neoplasms. Of the cases retrieved, 20 qualified as periductal stromal sarcoma and seven as periductal stromal hyperplasia. Patients with periductal stromal sarcoma ranged in age from 37 to 89 years (mean 55.3 years). The tumors measured 0.2-6.0 cm (mean 2.97 cm). Eighteen patients had excisional biopsies and two had partial mastectomies. Overall follow-up time ranged from 1 to 72 months (mean 25.3 months) with two patients (10%) showing recurrence or probable metastasis. The neoplastic cells of periductal stromal sarcoma were at least focally immunoreactive for CD34 (13 of 15), CD117 (6 of 15), less reactive for actin (HHF35, 2 of 15), and negative for estrogen and progesterone receptors. Periductal stromal sarcoma is a useful descriptive designation for generally low-grade biphasic tumors with sarcomatous stroma that do not have features of a phyllodes tumor. The development of focal phyllodes pattern in the recurrent tumor as well as development of a specific soft tissue sarcoma in one of the above cases suggest that some and possibly all periductal stromal sarcoma may evolve into a phyllodes tumor with time. Given the presence of infiltrative margins, excision with a rim of uninvolved tissue is required.
-
Most periductal stromal tumors are positive for CD34 based on above studies. Do some ones know phyllodes are negative for CD34? I have no personal experience about CD34 on phyllodes. If it is true CD34 can help to distinguish periductal stromal tumor from classic phyllodes.