| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- 上海市骨软组织病理读片会(2010#1)分享“上海中山医院”精彩病例(GICCS)
| 姓 名: | ××× | 性别: | 年龄: | ||
| 标本名称: | 读片会简介:上海市骨软组织病理读片会由上海肿瘤医院王坚教授和朱雄增教授提议创建于2007年。每季度举行一次, 每次讨论12例左右精彩的骨软组织疑难和罕见病例。2010年第一次读片会(2010-3-6)在上海瑞金医院举行,讨论的病例包括: 1)腹腔促纤维增生性小圆细胞肿瘤; 2)不典型纤维组织细胞瘤; 3)右心室转移性子宫平滑肌瘤; 4)神经纤维瘤病恶性变; 5)儿童多形性未分化肉瘤; 6)肾小球样血管瘤(POEMS综合症) 7)恶性颗粒细胞瘤; 8)男性会阴深部侵袭性血管粘液瘤; 9)乳腺癌放疗后不典型血管病变; 10)阴茎根部上皮样肉瘤; 11)食管肌层炎性肌纤维母细胞样肿瘤; 12)股骨颈软骨肉瘤3级,形态酷似软骨粘液样纤维瘤; 13)十二指肠-胰头部肿瘤(上海中山医院病理科-纪元教授提供) 现将上海中山医院病理科纪元教授提供的一例非常精彩的“十二指肠-胰头部肿瘤”提供大家欣赏。 | ||||
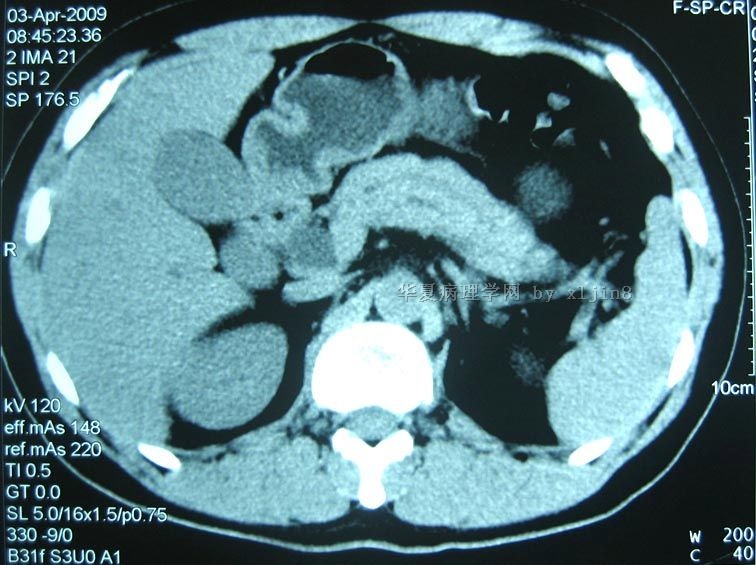
| 简要病史: | 男31岁,梗阻性黄疸2周。腹部CT示十二指肠降部占位。临床诊断:十二指肠间质瘤,行保留幽门十二指肠切除术。 手术中见肿瘤位于胰头和十二指肠降部,大小5x4cm,局部浸润横结肠系膜。
| ||||
| 肉眼检查: | 巨检:十二指肠粘膜下胆总管与胰腺间见灰褐色肿块,4x3cm,肿块质硬界不清。 | ||||
名称:图1
描述:图1
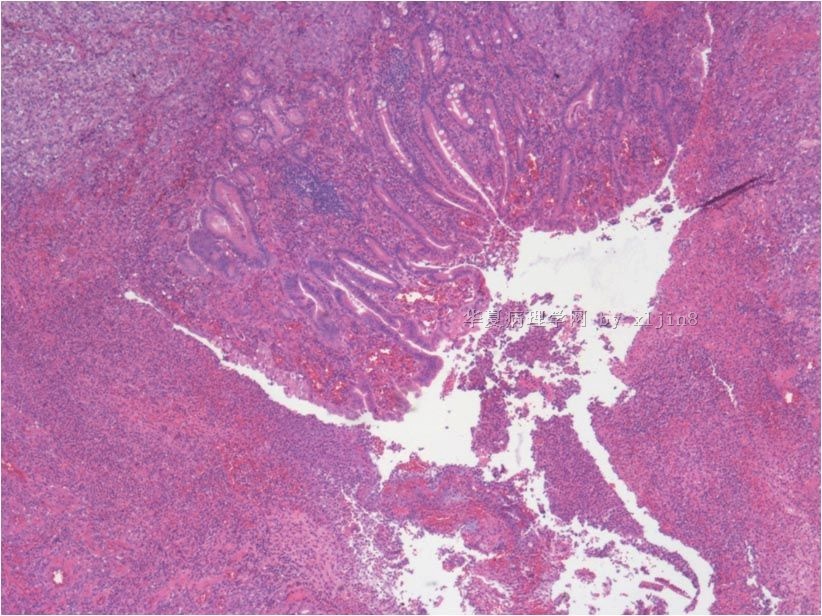
名称:图2
描述:图2
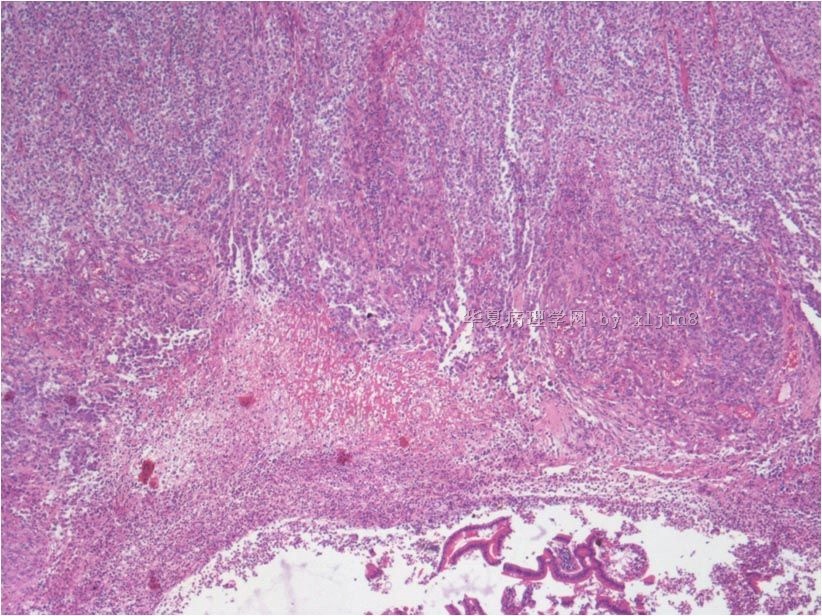
名称:图3
描述:图3
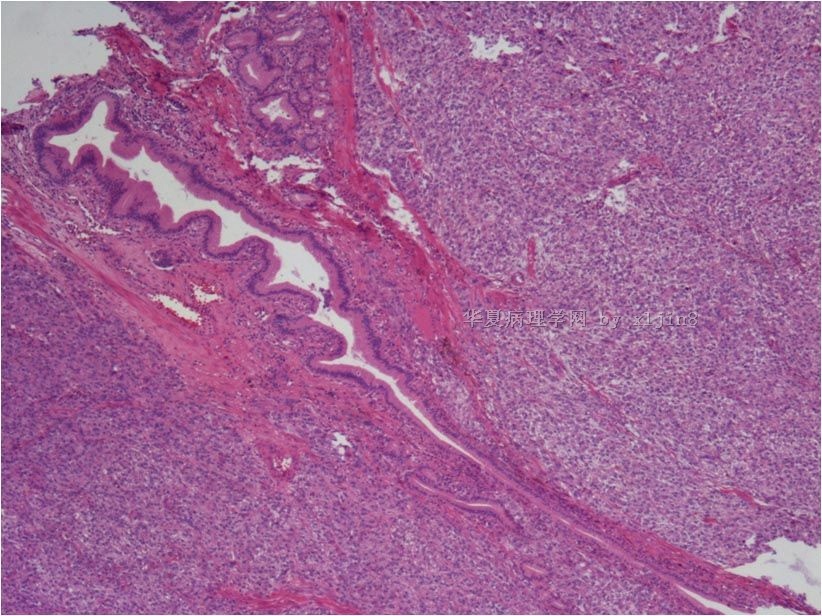
名称:图4
描述:图4
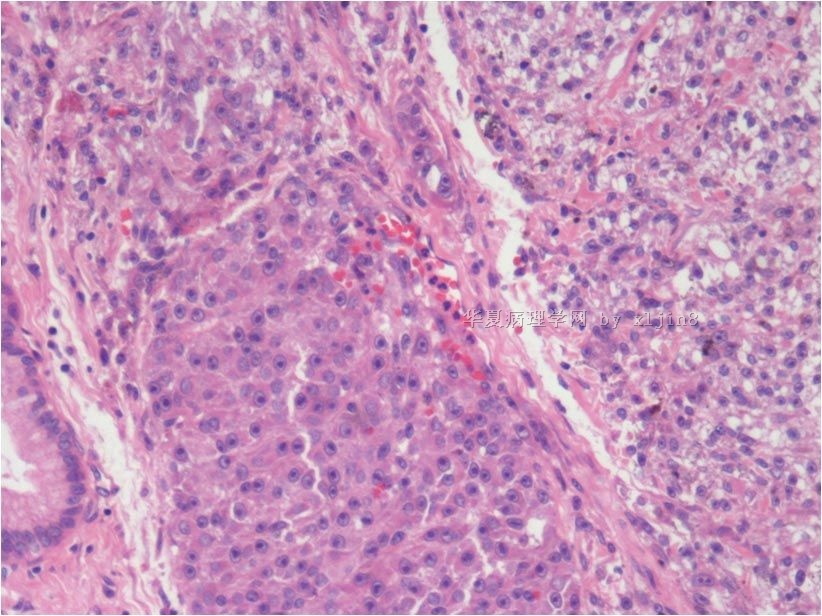
名称:图5
描述:图5
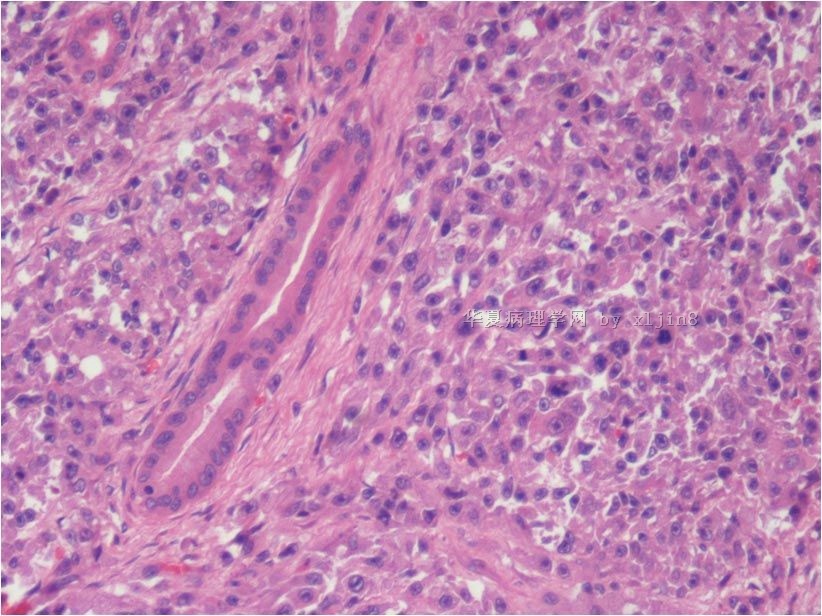
名称:图6
描述:图6
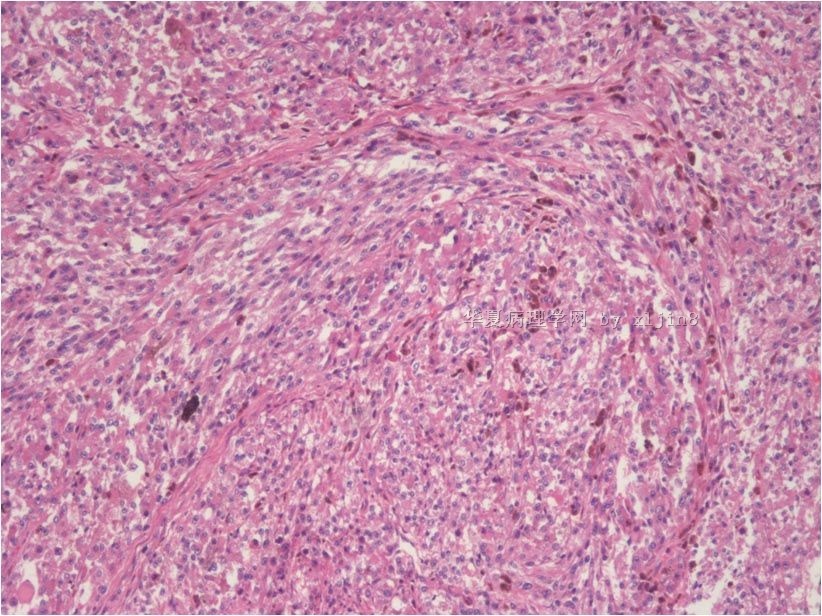
名称:图7
描述:图7
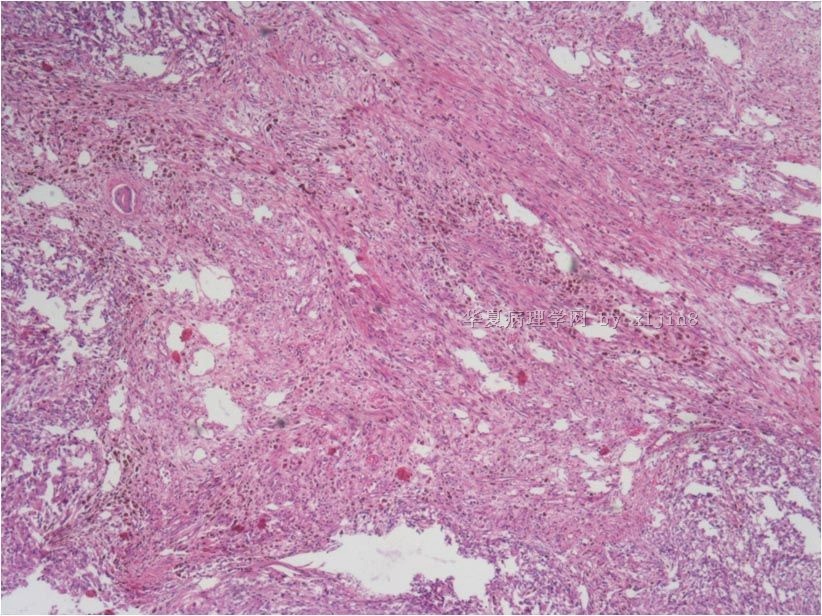
名称:图8
描述:图8
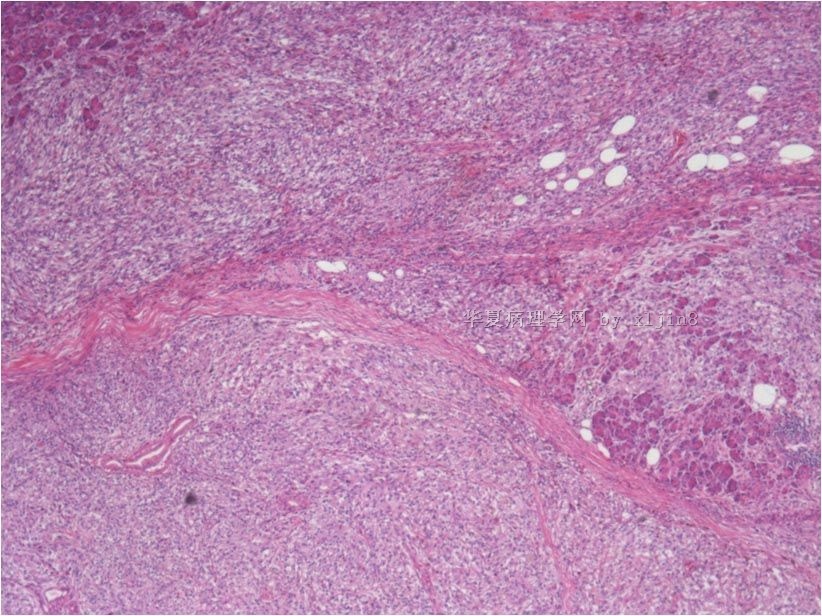
名称:图9
描述:图9
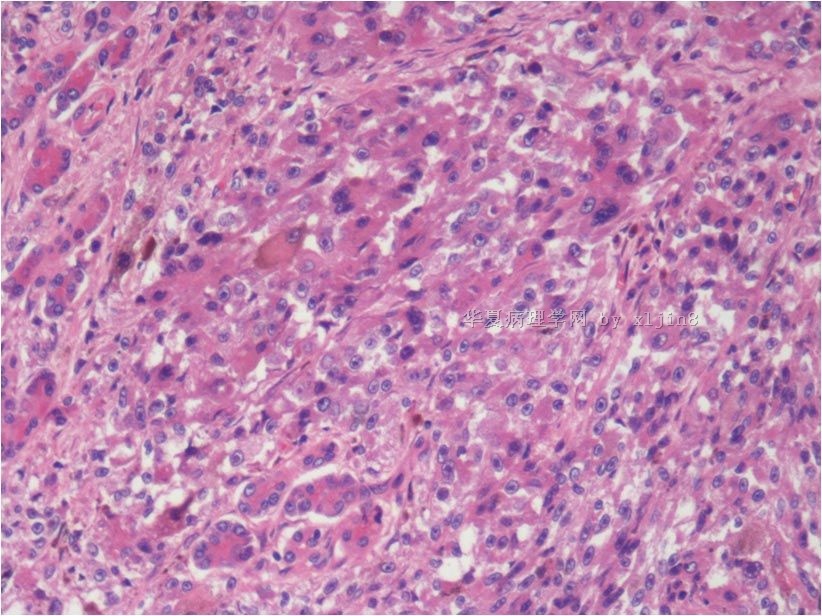
名称:图10
描述:图10
-
本帖最后由 于 2010-03-20 03:13:00 编辑

- xljin8
| 以下是引用xljin8在2010-3-14 21:00:00的发言:
1. Joo M,
Chang SH, Kim H, Gardner JM, Ro JY. Primary
gastrointestinal clear cell sarcoma: report of 2 cases, one case Department
of Pathology, Inje University Ilsan Paik Hospital, Goyang, South Korea. Clear cell
sarcoma (CCS) is a distinctive soft tissue sarcoma that shows melanocytic
differentiation. Primary gastrointestinal (GI) CCSs have been rarely reported,
but to our knowledge, no association between GI CCSs and immunoglobulin G4
(IgG4)-related sclerosing disease has been described in the literature. We experienced
2 cases of CCS that arose in the small intestine and metastasized to the liver.
2.Lyle PL, Amato CM, Fitzpatrick JE, Robinson WA. Gastrointestinal
melanoma or clear cell sarcoma? Molecular evaluation of 7 cases previously diagnosed as malignant melanoma. Clear cell
sarcoma (CCS) is a rare tumor classically associated with the tendons 3. Dow N,
Giblen G, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: differential
diagnosis. Semin Diagn Pathol. 2006;23:111-9. Division of
Gastrointestinal Pathology, Armed Forces Institute of Pathology, Availability
of KIT tyrosine kinase inhibitors for specific treatment of GISTs True smooth muscle tumors (rare in GI
tract except in esophagus and colon) can be separated from GISTs by the eosinophilic
tinctorial quality of tumor cells, positivity for smooth muscle markers, and
negativity for KIT. Desmoids
can form large GIST-like masses, but are composed of spindled or stellate- positive
non-GISTs include some sarcomas, especially angiosarcoma and Ewing sarcoma,
extramedullary myeloid tumor, seminoma, and a few carcinomas, notably small
cell carcinoma of lung. Spurious KIT-positivity, seen with some polyclonal KIT
antibodies, has been a source of confusion leading to probable false-positive
results in fibroblastic tumors and occasional other sarcomas, such as
leiomyosarcomas. Integration of histological features with carefully standardizedimmunohistochemistry,
supported by KIT and PDGFRA mutation analysis, is the cornerstone of
state-of-the art differential diagnosis of GIST. 4. Agaimy
A, Wünsch PH.Perivascular epithelioid
cell sarcoma (malignant PEComa) of the ileum. Pathol Res Pract.
2006;202:37-41. Institut
für Pathologie, Klinikum Nürnberg, Prof. Ernst-Nathan-Strasse 1, 90419 Nürnberg,
Germany. abbas.agaimy@klinikum-nuernberg.de Epithelioid
angiomyolipoma (AML) is the prototype of a heterogeneous group of lesions
characterized by the presence of HMB-45 positive cells with clear cytoplasm,
perivascular distribution, and combined myomelanocytic features,so-called
perivascular epithelioid cells (PECs). These lesions are being increasingly
referred to as PEComas. PEComas have been reported at diverse anatomic sites,
but mainly in the abdominopelvic cavity and rarely in parenchymatous organs,
skin, and soft tissues. Gastrointestinal (GI) PEComas are exceptionally rare,
with less than 10 cases documented so
far. Rare examples of PEComas with pleomorphic histology could have been
misinterpreted as unusual variants of carcinoma or sarcoma. To make a
contribution to the differential diagnosis of difficult-to-classify pleomorphic
GI sarcomas, we report on a malignant pleomorphic neoplasm with features of
PEComa involving the terminal ileum in a 63-year-old woman. Fourteen months
after resection of the primary tumor, a huge abdominopelvic recurrence was
successfully resected, but no distant metastases were detected. The
differential diagnosis and malignancy criteria of GI PEComas will be discussed. 5. Covinsky
M, Gong S, Rajaram V, Perry A, Pfeifer J. EWS-ATF1 fusion transcripts in
gastrointestinal tumors previously diagnosed as malignant melanoma. BACKGROUND:
Clear cell sarcoma (CCS) is classically a deep soft tissue tumor associated
with tendons or aponeuroses, although cases of primary CCS of the gastrointestinal
(GI) tract have recently been reported. Because it is difficult to distinguish
CCS from metastatic melanoma based on morphology, immunohistochemical profile,
and ultrastructural features, it is possible that some GI tumors diagnosed as
metastatic melanoma actually represent primary GI CCS. Because the EWS-ATF1
fusion transcript and the associated t(12;22)(q13;q12) translocation occur in
CCS but not cutaneous melanoma, we investigated the use of molecular-based
testing for discriminating CCS from metastatic melanoma (MM) in GI tumors. METHODS:
Patients with GI tumors diagnosed as MM were identified from departmental
files. The tumors were tested for EWS-ATF1 fusion transcript by RT-PCR and fort(12;22)(q13;q12)
by fluorescence in situ hybridization. |

- xljin8
-
joule_0908 离线
- 帖子:47
- 粉蓝豆:3205
- 经验:118
- 注册时间:2009-07-23
- 加关注 | 发消息