| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- B1-2(华中科技大学同济医学院附属同济医院)_已点评
-
本帖最后由 于 2009-11-20 23:12:00 编辑
提供一些资料,来自Rosen's Breast Pathology 3ed_2009
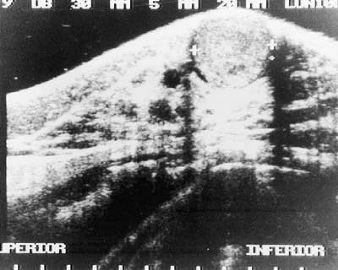
Fig. 6.1. Adenomyoepithelioma. This ultrasound image shows a well-circumscribed, slightly inhomogeneous mass in the subareolar region.
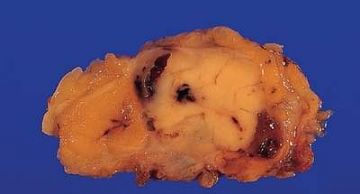
Fig. 6.2. Adenomyoepithelioma. This bisected tumor is circumscribed and has a nodular architecture. The tumor measures about 2 cm.
Fig. 6.3. Adenomyoepithelioma. Two different gross specimens are illustrated. Skin is present on the upper borders of the specimens. A: Multiple transverse sections of a mastectomy extensively involved by a multicystic adenomyoepithelioma with papillary areas. B: A solid and cystic multinodular adenomyoepithelioma that measured about 7 cm in greatest diameter is shown in transverse sections.
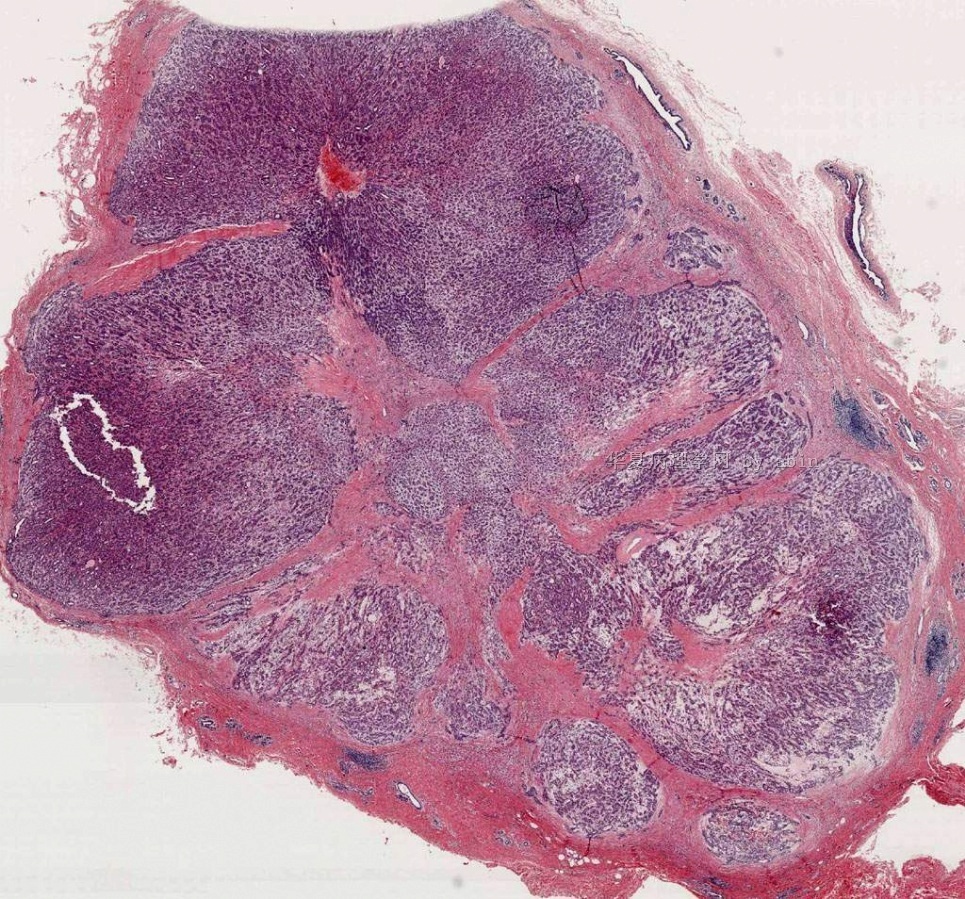
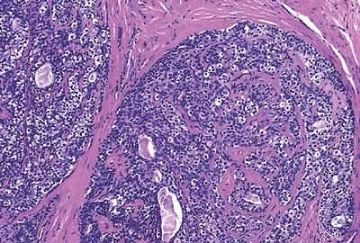
Fig. 6.4. Adenomyoepithelioma. A whole mount histologic section in which multiple nodules surround a sclerotic core. There are remnants of ducts with papillary elements (arrows).
Fig. 6.5. Adenomyoepithelial hyperplasia of lobules. A: A group of lobules in which hyperplastic myoepithelial cells surround the lobular glands, duplicating the pattern found in an adenomyoepithelioma. B: Aggregated lobules with very prominent myoepithelial hyperplasia forming a tumor.
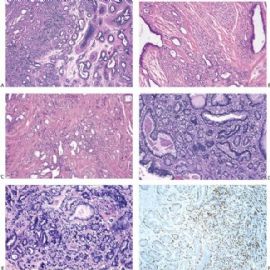
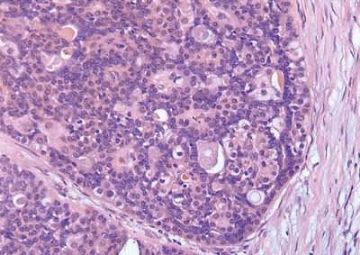
Fig. 6.6. Adenomyoepithelioma with adenosis structure. A: Myoepithelial hyperplasia shown here in sclerosing adenosis is composed of small cells with clear cytoplasm (left). Sclerosing adenosis without myoepithelial hyperplasia is shown on the right. B,C: Myoepithelial cells with clear cytoplasm in sclerosing adenosis. D,E: Adenomyoepithelioma with adenosis structure and myoepithelial hyperplasia composed of epithelioid cells with clear cytoplasm around the perimeter of adenosis glands. F: Myoepithelial cells are demonstrated in the tumor by the P63 immunostain (avidin-biotin).
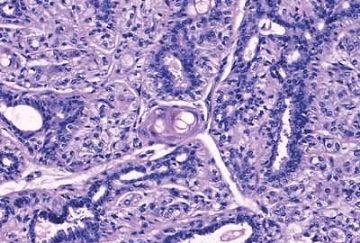
Fig. 6.7. Adenomyoepithelioma with microglandular adenosis (MGA) pattern. A: Adenosis glands with an infiltrative growth pattern that resembles MGA in fat. Clear cells represent the myoepithelial component. B: Myoepithelial cell nuclei in A are highlighted by the P63 immunostain (avidin-biotin). C: A region with MGA-like infiltrative growth around ducts (above) and nodular growth (below). D: Myoepithelial cell hyperplasia in C is highlighted here by the P63 immunostain (avidin-biotin).
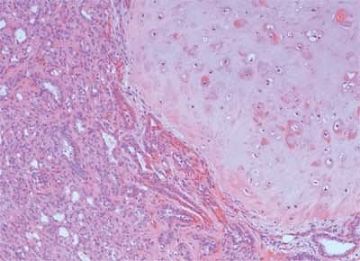
Fig. 6.8. Adenomyoepithelioma. A: The characteristic growth pattern in which cords and irregular aggregates of epithelial cells are separated by bands of fibrovascular stroma. Some glandular lumens contain secretion. B: Myoepithelial cells with clear cytoplasm are aligned along the serrated outer edges of epithelial cords.
Fig. 6.9. Adenomyoepithelioma. A: Prominent myoepithelial cell hyperplasia is present between glands. B: Myoepithelial cells fill the spaces between glands. C: An area composed entirely of epithelioid myoepithelial cells next to glands.
Fig. 6.10. Adenomyoepithelioma. Two areas in the same tumor are shown. A: Balanced glandular and myoepithelial proliferation. B: Epithelioid clear cell myoepithelial hyperplasia has displaced glandular structures.

华夏病理/粉蓝医疗
为基层医院病理科提供全面解决方案,
努力让人人享有便捷准确可靠的病理诊断服务。
-
本帖最后由 于 2009-11-20 23:13:00 编辑
Fig. 6.11. Adenomyoepithelioma with sclerotic stroma. A: Conventional adenomyoepithelioma structure. B,C: A trabecular growth pattern created by sclerotic stroma in which there are foci of collagenous spherulosis (C).
Fig. 6.12. Adenomyoepithelioma. Epithelioid myoepithelial cells, many with clear cytoplasm, are arranged in a trabecular pattern.
Fig. 6.13. Adenomyoepithelioma. In this view, myoepithelial cells with clear cytoplasm have formed alveolar clusters between glands.
Fig. 6.14. Adenomyoepithelioma. Clusters of myoepithelial cells with pink cytoplasm are evident in the epithelium of the solid papillary hyperplasia in a duct at the margin of an adenomyoepithelioma.
Fig. 6.15. Apocrine metaplasia in an adenomyoepithelioma. A: Apocrine epithelium is evident in the glands. Spindly myoepithelial cells are present. B: Atypical apocrine metaplasia with nuclear atypia surrounded by epithelioid myoepithelial cells.
Fig. 6.16. Sebaceous metaplasia in an adenomyoepithelioma. Sebaceous differentiation manifested by a discrete, round group of cells with clear, vacuolated cytoplasm.
Fig. 6.17. Squamous metaplasia in an adenomyoepithelioma. Squamous metaplasia in the form of a keratin pearl is seen in the midst of myoepithelial cells with clear cytoplasm. Squamous and sebaceous metaplasia appears to originate in the myoepithelial cells.
Fig. 6.18. Cartilaginous metaplasia in an adenomyoepithelioma.
Fig. 6.19. Adenomyoepithelioma with mucoepidermoid differentiation. A: A part of the tumor with the typical adenomyoepithelioma growth pattern. B,C: Mucoepidermoid differentiation in a cystic portion of the tumor. D,E: Mucinous secretion in glands. F: Squamous metaplasia with glandular elements. G–I: Adenomyoepithelioma with mucin formed by epithelioid myoepithelial cells. Vacuolated epithelial cells in the papillary tumor (G,H) containing mucin, which is stained magenta by the mucicarmine stain (I). J: Collagenous spherulosis in a papillary adenomyoepithelioma. The basophilic material in spherules surrounded by a distinct basement membrane resembles mucin.
Fig. 6.20. Adenomyoepithelioma with infarction. A: Calcification in an infarcted lesion. B–D: Infarcted adenomyoepithelioma with squamous metaplasia (B). Myoepithelium is demonstrated in infarcted (C) and intact (D) regions with the CD10 immunostain (avidin-biotin).

华夏病理/粉蓝医疗
为基层医院病理科提供全面解决方案,
努力让人人享有便捷准确可靠的病理诊断服务。
-
本帖最后由 于 2009-11-20 23:44:00 编辑
Fig. 6.21. Cystic adenomyoepithelioma. A: At low magnification, nodules of adenomyoepitheliomatous growth protrude from the surface of the cyst, which is otherwise lined by ordinary duct epithelium. B: An adenomyoepitheliomatous nodule at the periphery of a duct.
Fig. 6.22. Adenomyoepithelioma. A,B: This lesion has a conspicuous myoid component composed of spindle cells with deeply eosinophilic cytoplasm between the glands.
Fig. 6.23. Adenomyoepithelioma. A: Two oval glands in the center and one round gland (lower left) are surrounded by a spindly myoepithelial proliferation. B: An area with pure spindle cell myoepithelium.
Fig. 6.24. Adenomyoepithelioma. A: Spindle-shaped myoid myoepithelial cells in a palisade arrangement. B: Nuclear atypia in myoid myoepithelial cells. C: Nuclear atypia in epithelioid myoepithelial cells.
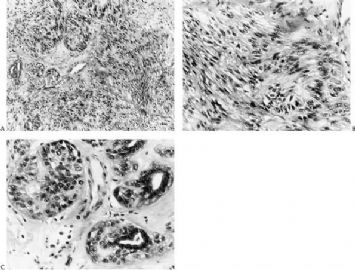
Fig. 6.25. Malignant adenomyoepithelioma with epithelial and myoepithelial carcinoma. A: Neoplastic proliferation of epithelial and myoepithelial cells with mitotic activity. B: Comedonecrosis is present in the epithelial component (upper right). Mitotic activity is present.
Fig. 6.26. Myoepithelial carcinoma arising in adenomyoepithelioma. A: Overgrowth of myoepithelial cells in the primary tumor. B: An area in (A) with mitotic activity (arrows) in myoepithelial cells that have prominent nucleoli. C: Part of the primary tumor composed almost entirely of atypical myoepithelial cells with an epithelial phenotype. D: Recurrent tumor growing as myoepithelial carcinoma. The carcinoma cells have prominent nucleoli and frequent mitoses (arrows). E–I: Images are from a single tumor. Adenomyoepithelioma with myoepithelial hyperplasia (E). Pronounced hyperplasia of atypical myoepithelial cells is shown displacing glandular epithelial cells (F). A region in which there is nearly complete overgrowth of myoepithelial cells retaining the fundamental adenomyoepithelial architecture (G). Myoepithelial carcinoma in enlarged alveolar nests (H). Invasive myoepithelial carcinoma (I,J).
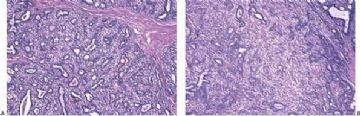
Fig. 6.27. Mixed tumor. A: Collagenized matrix adjacent to glandular elements with an adenosis pattern. B: Carti-laginous differentiation in the stroma. C: Sebaceous metaplasia of the epithelium.
Fig. 6.28. Mixed tumor. Part of the tumor is composed of a myoepithelial proliferation in a myxoid matrix that resembles a cellular mixed tumor.
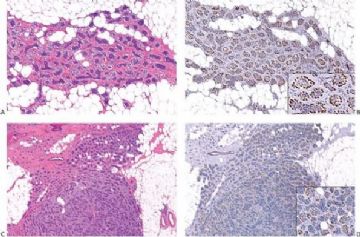
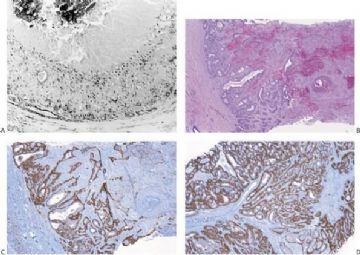
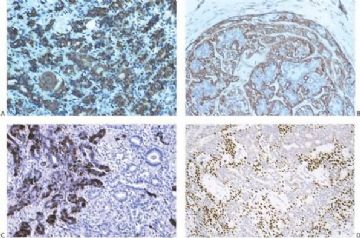
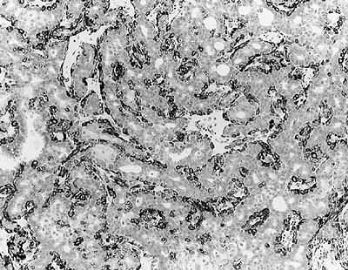
Fig. 6.29. Adenomyoepithelioma immunoreactivity. A: The epithelial cells including a keratin pearl in the lower left corner are stained with anti-keratin antibody. The myoepithelial cells are not immunoreactive (anti-AE1/3, avidin-biotin). B: Myoepithelial cells are immunoreactive for actin, which does not stain the epithelial cells (anti-smooth muscle actin; avidin-biotin). C: Focal S-100 immunoreactivity in glandular epithelial cells (anti-S-100, avidin-biotin). D: Nuclear reactivity for P63 is localized in myoepithelial cells (avidin-biotin).
Fig. 6.30. Adenomyoepithelioma. Immunoreactivity for actin is present in myoepithelial cells bordering on the glandular proliferation (anti-smooth muscle actin, avidin-biotin).

华夏病理/粉蓝医疗
为基层医院病理科提供全面解决方案,
努力让人人享有便捷准确可靠的病理诊断服务。
-
本帖最后由 于 2009-11-20 23:49:00 编辑
Adenomyoepithelioma
The first full description of adenomyoepithelioma of the breast was published in 1970 by Hamperl (13). With the exception of 4 studies consisting, respectively, of 6, 13, 18 and 27 cases (14,15,16,17), reports of these lesions have been case studies (4,18,19,20,21,22,23,24,25,26).
Clinical Presentation
Virtually all patients have been women ranging in age from 27 to 80 years (average age, 58 years) in one series of 18 cases (16), and from 26 to 82 years (average 63 years) among patients in another series and in various case reports. Most patients present with a solitary unilateral painless mass located in a peripheral portion of the breast. Occasionally the lesions have been found centrally or near the areola (17,18,21,27) (Fig. 6.1). Nipple discharge, pain, and tenderness are infrequent (17). The tumor has reportedly been palpable for a year or more (16,17,23) before excision. Most patients describe recent onset. The lesion was observed by mammography 2 years before excision in one case (16) and the mammographic findings were considered to be suspicious in some patients (16,20). Nonpalpable adenomyoepitheliomas measuring 1 to
Berna et al. (29) described a partially cystic, 2.5-cm, circumscribed adenomyoepithelioma that arose in an 84-year-old man. The tumor was histologically benign, with immunoreactivity for actin and cytokeratin. Adenomyoepithelioma does not have a predilection for either breast. Data on family history of breast carcinoma or of other significant diseases are rarely mentioned in published case reports, and the information available does not suggest any associated condition. Biochemical estrogen receptor (ER) and progesterone receptor (PR) analysis performed on some tumors (16,30) yielded the following results: ER+ (171 fmol); ER+ (44.7 fmol), PR–; ER+ (23 fmol), PR–; ER+ (13 fmol), PR–; ER–, PR–(3 cases). Another lesion also had positive (37 fmol) ERs (25). When studied by immunohistochemistry, the glandular epithelial component displays nuclear reactivity for ERs in most cases. PR reactivity is often absent. Hormone receptors are almost always not present in the myoepithelial component.
Gross Pathology
The reported grossly measured size of adenomyoepitheliomas varied from 0.5 to
Microscopic Pathology
A spectrum of histologic patterns is encountered among these tumors and even within different portions of a single lesion. Factors that influence these variations include the proportions of proliferating glandular and myoepithelial cells, the degree to which myoepithelial cells have a spindle or polygonal configuration, the prominence of papillary components, and the extent of fibrosis.
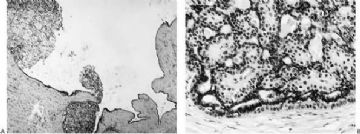
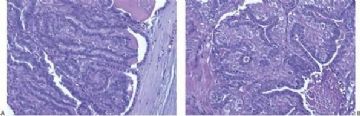
Microscopically, most adenomyoepitheliomas are circumscribed and composed of aggregated nodules (Fig. 6.4). The majority of adenomyoepitheliomas are variants of intraductal papilloma, but a small number of these tumors appear to arise from a lobular proliferation or adenosis (Figs. 6.5 and 6.6). Clear cell epithelioid myoepithelial hyperplasia in an adenomyoepithelioma with adenosis structure could be mistaken
for invasive carcinoma in a small biopsy sample (Fig. 6.6). The most unusual and exceedingly rare adenosis variant of adenomyoepithelioma has an infiltrative growth pattern that resembles microglandular adenosis (MGA) (Fig. 6.7). However, MGA is characterized by absence of myoepithelium (see Chapter 7). Some adenomyoepithelioma nodules consist of a single, compact proliferation of epithelial and myoepithelial cells, but most lesions have one or more nodules in which there is a focal papillary growth pattern. Sometimes the papillary intraductal component extends into ducts outside the gross tumorous lesion. This characteristic may be responsible for recurrence after seemingly adequate excision. A minority of adenomyoepitheliomatous tumors consist largely or entirely of intraductal papillary elements, an observation that supports the conclusion that these lesions are variants of intraductal papilloma. In this respect adenomyoepithelioma is closely related to ductal adenoma (20,31,32) and pleomorphic adenoma (33,34). Foci of adenomyoepithelioma can frequently be detected in tubular adenomas. They are less apparent in mammary pleomorphic adenomas, in which an associated papilloma is usually evident.
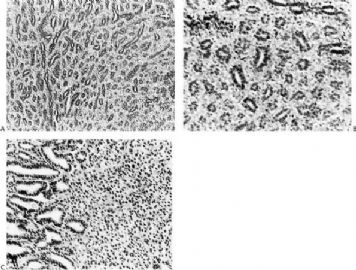
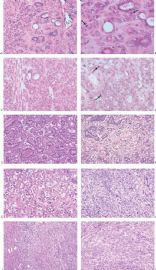
The basic structural unit of the adenomyoepithelioma is a small round or oval glandular lumen encompassed by cuboidal epithelial cells. At the periphery of the glands are polygonal or spindle-shaped myoepithelial cells with clear cytoplasm and a basement membrane (Fig. 6.8). The most common microscopic pattern, referred to as the tubular type of adenomyoepithelioma, features a balanced proliferation of round, oval, or tubular glandular elements invested by of polygonal myoepithelial cells with clear cytoplasm. In some tumors, myoepithelial cells with clear cytoplasm are more numerous than epithelial cells, resulting in zones virtually devoid of glands (Figs. 6.9 and 6.10). Other lesions feature myoepithelial cells, which proliferate in broad bands and trabeculae separated by strands of fibrovascular stroma (Figs. 6.11 and 6.12). Collagenous spherulosis can develop in the stroma (Fig. 6.11). The contrast between the dark-staining cytoplasm of glandular cells and the pale cytoplasm of myoepithelial cells is striking (Fig. 6.13). Small glandular lumens formed within the epithelial areas may have a pattern reminiscent of an endocrine neoplasm. In papillary regions, distinct polygonal myoepithelial cells accompany the epithelium in its various branches and ramifications (Fig. 6.14).
The epithelial cells tend to have sparse, darkly stained cytoplasm and hyperchromatic nuclei. In some cases, this produces a plasmacytoid appearance. Apocrine metaplasia may be encountered in the glandular epithelium, particularly in papillary areas (Fig. 6.15). The apocrine epithelium can be cytologically atypical. Glands exhibiting sebaceous metaplasia are variably present (Fig. 6.16), and focal squamous metaplasia (Fig. 6.17) occurs in a minority of cases. Cartilaginous metaplasia of the stroma is rarely seen (Fig. 6.18). A previously undescribed adenomyoepithelioma with mucoepidermoid differentiation has been encountered (Fig. 6.19). Cytologic atypia and mitotic activity are very infrequent or absent from lesions in which the myoepithelial cells retain a polygonal configuration and there are conspicuous papillary areas. Stromal and myoepithelial elements may have an adenoid cystic pattern. This may be manifested by the presence of collagenous spherulosis (27) (Fig. 6.19). Calcifications are occasionally present. Some tumors develop central fibrosis or ischemic necrosis leading to calcification (Fig. 6.20). The cystic papillary type of adenomyoepithelioma is very uncommon (Fig. 6.21).
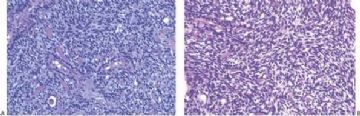
The extent to which myoepithelial cells assume a spindly, myoid shape varies greatly. Tumors that are composed entirely of such elements with no identifiable epithelial cells are classified as myoepitheliomas and discussed separately in this chapter. However, many adenomyoepitheliomas have only limited foci of spindle cell myoid growth (Fig. 6.22). Usually the myoid areas have a mixture of spindle and polygonal cells. The glandular component may be intermixed with myoid areas or it may appear to be largely independent (Fig. 6.23). Palisading of spindle cells and alveolar clustering of polygonal myoepithelial cells are common myoid patterns. The latter configuration has been referred to as the lobulated type of adenomyoepithelioma (17). A myoepithelial tumor of the male breast composed of spindle cells arranged in a storiform pattern was described by Tamura et al. (35). Despite the spindle cell phenotype, the neoplasm was most strongly reactive for cytokeratin and S-100, with little staining for actin and no vimentin reactivity. Cytologic atypia of myoepithelial cell nuclei may be encountered in lesions where there is a tendency to form spindle cells resulting in a distinctly myoid appearance (Fig. 6.24). In these foci, atypical features include scattered mitotic figures, nuclear pleomorphism, hyperchromasia, and occasional multinucleated cells. Myoid hyperplasia may give rise to areas with leiomyomatous features (5,28) and rarely this process produces leiomyosarcoma (5). Cytologic atypia and mitotic activity have also been described in the lobulated type of adenomyoepithelioma (17).
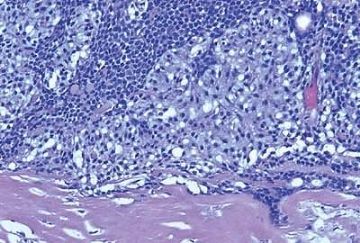
Origin of a malignant neoplasm in an adenomyoepithelioma resulting in a malignant adenomyoepithelioma has only very rarely been documented. Malignant transformation may be limited to either the epithelial or myoepithelial component, or both elements may be involved. Histologic evidence of malignant growth includes mitotic activity, necrosis, cellular pleomorphism, overgrowth of myoepithelium or epithelium, and invasion at the periphery of the tumor. Many of the lesions
purported to be malignant adenomyoepitheliomas have featured a spindle cell component, and in some instances, the distinction from metaplastic carcinoma is not clear (36). Some lesions were reported to have recurred locally (15,37,38,39), whereas others resulted in metastases and a fatal outcome (38,39,40,41). In one fatal case, the malignant component, described as “undifferentiated,” lacked reactivity for cytokeratin and actin (41), but in other tumors, these cytoskeletal components were immunohistochemically detectable (38,40). Malignant adenomyoepitheliomas with a biphasic growth pattern at the primary site and in metastases have also been reported (24) (Fig. 6.25). Myoepithelial carcinoma with an epithelial phenotype can arise in an adenomyoepithelioma (Fig. 6.26). These neoplasms are characterized by overgrowth of the myoepithelial component exhibiting mitotic activity and cytologic evidence of carcinoma, often with foci of necrosis (38). Simpson et al. (39) described a malignant adenomyoepithelioma with carcinomatous and osteogenic elements. A case report by Jones et al. (42) documents a 3-cm malignant adenomyoepithelioma, which had a substantial malignant spindle cell component with mitoses and necrosis. The tumor gave rise to multiple liver metastases composed of spindle cells. Comparative genomic hybridization (CGH) analysis revealed progressively more genetic alterations between the glandular and spindle cell elements of the primary tumor and the liver metastases.
The group of tumors sometimes referred to as mixed tumors or pleomorphic adenomas of the breast are variants of intraductal papilloma and adenomyoepithelioma. These tumors are more frequently located in the subareolar region than in the periphery of breast, and probably arise from large lactiferous ducts (43,44). Most are solid, circumscribed tumors, but an intracystic mural lesion has been described (44). Remnants of the underlying epithelial lesion usually with myoepithelial cell hyperplasia can be found in almost all of these tumors (43,45). A characteristic feature of many mixed tumors in the breast is the formation of collagenized myxoid matrix that is frequently converted into cartilage (Fig. 6.27). Calcification and ossification occur in some mixed tumors as well. Various proliferative patterns are found in the lesions, including foci that resemble cellular mixed tumor, and a distinct papillary component (Fig. 6.28). The epithelium may develop squamous and sebaceous metaplasia similar to the metaplastic changes that occur in conventional adenomyoepitheliomas (Fig. 6.27). The cytologic findings in an FNA specimen may suggest a phyllodes tumor when numerous bipolar spindle cells are present or metaplastic carcinoma if the aspirate contains abundant metachromatic stroma (46).
Immunohistochemistry
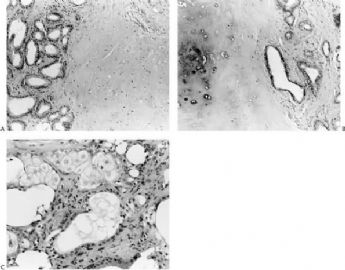
By and large the diverse cellular components exhibit expected histochemical properties. Glands may contain periodic acid–Schiff (PAS)- or mucicarmine-positive secretion, but intracytoplasmic secretion is rarely detectable. The secretion is also positive for carcinoembryonic antigen (CEA) in most cases. The cytoplasm of glandular cells tends to be strongly reactive with antibodies to cytokeratin, and the luminal surfaces of these cells are positive for epithelial membrane antigen (Fig. 6.29). Although most of the epithelial cells are S-100 negative, small groups may be strongly reactive for this antigen (Fig. 6.29).
Polygonal and spindle myoepithelial cells are not reactive for epithelial membrane antigen (EMA) or CEA and are variably reactive for cytokeratin. The distribution and intensity of staining obtained with anti-actin antibodies is heterogeneous. Reactivity is most pronounced for smooth muscle myosin heavy chain. Anti-actin staining tends to be more conspicuous in spindle than in clear polygonal cells, and no reactivity is seen in epithelial cells (Figs. 6.29 and 6.30). Some myoepithelial cells are S-100 positive in virtually all tumors, but the intensity and uniformity of reactivity varies considerably.
There does not appear to be a consistent relationship between staining with anti-actin and anti-S100 antibodies in myoepithelial cells. Because S-100 reactivity is expressed by glandular and myoepithelial cells it is not a specific marker for the latter cell type in adenomyoepitheliomas (47).
Immunostain markers that are relatively specific for myoepithelial cells include CK5, CD10, myosin, calponin, and P63. P63 (Figs. 6.6 and 6.29) is especially helpful because of its nuclear localization that precludes cytoplasmic cross-reactivity which occurs sporadically with markers such as myosin, calponin, smooth muscle actin (SMA), and CD10. The immunophenotype of neoplastic myoepithelial cells is sometimes different from that of their normal counterpart, and they are not necessarily reactive for all markers. Therefore, a panel of immunostains should be used to minimize the likelihood of failing to detect the presence of myoepithelial cells. Maspin (48,49) and p75 neurotrophin receptor (p75NTR) (50) are presently additional investigational markers for myoepithelial cells.
Electron Microscopy
Several papers have described the ultrastructural features of individual lesions classified as adenomyoepitheliomas (3,14,18,25,26,30,51). In general, these case reports have documented the presence of epithelial and myoepithelial components in the lesions. Short microvilli are present at the luminal surfaces of glandular cells, which are joined at the luminal edges by tight cell junctions. The cytoplasm contains scattered mitochondria as well as smooth and rough endoplasmic reticulum. Polygonal and spindle-form myoepithelial cells have desmosomes that may be well or poorly formed, as well as interdigitating cell processes. Keratin and actin cytofilaments are prominent in the cytoplasm of myoepithelial cells, sometimes arranged in perinuclear bundles or peripheral arrays. Fusiform densities or condensation zones are commonly found along these arrays of cytofilaments. Distinct basal lamina material is found around and among the myoepithelial cells.
Cytology
Aspiration cytology of a typical adenomyoepithelioma yields a cellular specimen composed of relatively large epithelial cells distributed in clusters or separately (30,52). The epithelial cells tend to have deeply staining cytoplasm and prominent round to oval nuclei that may be eccentrically placed. Spindle cells and myoid fibrillary material are variably present in the background (52). The cytologic features may suggest a diagnosis of carcinoma (12,53,54).
Prognosis and Treatment
The majority of adenomyoepitheliomas are benign tumors that can be treated by local excision (16). Local recurrence has been reported, usually more than 2 years after the initial excision (4,15,17). In two cases, two (16) and three (4) episodes of recurrence, may be attributed to incomplete excision. The multinodular character of the lesion and peripheral intraductal extension in one of these patients were probably contributory factors (16). There is no evidence that cytologic atypia or the proportions of spindle and polygonal myoepithelial cells are related to the risk for local recurrence. Carcinoma may be detected as a separate lesion coincidentally or subsequent to excision of an adenomyoepithelioma (17). Adenocarcinoma was reportedly found in one recurrent adenomyoepithelioma (17).
Re-excision may be performed when the tumor is incompletely excised, especially for multinodular lesions with peripheral intraductal extension. Mastectomy, breast irradiation, and axillary dissection are not appropriate treatment for benign adenomyoepitheliomas but may be indicated for those exceptional patients who have malignant tumors.
References
1. Gusterson BA, Warburton MJ, Mitchell D, et al. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res 1982;42:763–770.
2. Cameron NM, Hamperl H, Warambo W. Leiomyosarcoma of the breast originating from myoepithelium (myoepithelioma). J Pathol 1974;114: 89–92.
3. Eusebi V, Casadei GP, Bussolati G, et al. Adenomyoepithelioma of the breast with a distinctive type of apocrine adenosis. Histopathology 1987;11:305–315.
4. Young RH, Clement PB. Adenomyoepithelioma of the breast. A report of three cases and review of the literature. Am J Clin Pathol 1988;89: 308–314.
5. Franke WW, Schmid E, Freudenstein C, et al. Intermediate sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol 1980;36: 633–654.
6. Joshi K, Smith JA, Perusingle N, et al. Cell proliferation in human mammary epithelium. Differential contribution of epithelial and myoepithelial cells. Am J Pathol 1986;124:199–206.
7. Rudland PS. Histochemical organization and cellular composition of ductal buds in developing human breast: Evidence of cytochemical intermediates between epithelial and myoepithelial cells. J Histochem Cytochem 1991;39:1471–1484.
8. O'Hare MJ, Ormerod MO, Monaghan P, et al. Characterization in vitro of luminal and myoepithelial cells isolated from the human mammary gland by cell sorting. Differentiation 1991;46:209–221.
9. Dardick I, Van Nostrand AWP. Myoepithelial cells in salivary gland tumors revisited. Head Neck Surg 1985;7:395–408.
10. Kahn HJ, Baumol R, Marks A, et al. Myoepithelial cells in salivary gland tumors: An immunohistochemical study. Arch Pathol Lab Med 1985; 109:190–195.
11. Zhang C, Quddus R, Sung CJ. Atypical adenomyoepithelioma of the breast: Diagnostic problems and practical approaches in core needle biopsy. Breast J 2004;10:154–155.
12. Chang A, Bassett L, Bose S. Adenomyoepithelioma of the breast: A cytologic dilemma. Report of a case and review of the literature. Diagn Cytopathol 2002;26:191–196.
13. Hamperl H. The myoepithelia (myoepithelial cells): Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol 1970;53:161–213.
14. Decorsiere JB, Thibaut I, Bouissou H. Les proliférations àdeno-myoepithéliales du sein. Ann Pathol 1988;8:311–316.
15. Loose JH, Patchefsky AS, Hollander IJ, et al. Adenomyoepithelioma of the breast. A spectrum of biologic behavior. Am J Surg Pathol 1992; 16:868–876.
16. Rosen PP. Adenomyoepithelioma of the breast. Hum Pathol 1987;18: 1232–1237.
17. Tavassoli FA. Myoepithelial lesions of the breast. Myoepitheliosis, adenomyoepithelioma, and myoepithelial carcinoma. Am J Surg Pathol 1991;15:554–568.

华夏病理/粉蓝医疗
为基层医院病理科提供全面解决方案,
努力让人人享有便捷准确可靠的病理诊断服务。
-
wangdingding 离线
- 帖子:1474
- 粉蓝豆:98
- 经验:6042
- 注册时间:2006-10-19
- 加关注 | 发消息