| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
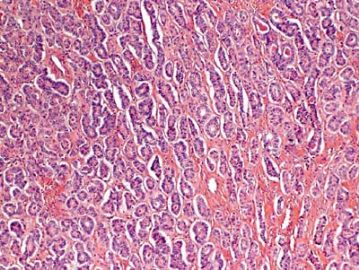
- 卵巢Sertoliform (or sex cord-like) variant of 子宫内膜样癌 (cqz-3)
Agree. In addition, the upper left photo is typical adenocarcinoma, to my eyes
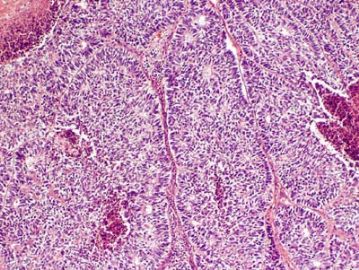
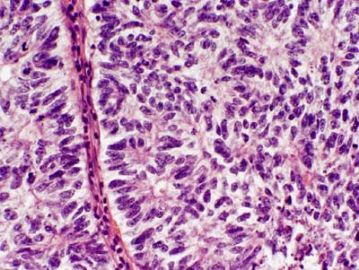
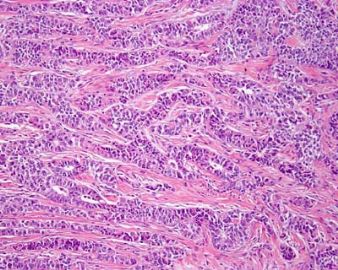
The upper right and lower left appear similar, same type with low and high power? The right lower looks different again. Could this be a collision tumor of different kinds?
Thank above a lot of very good analysis.
Summary above discussion:
Differential diagnosis (DDX):
1. Sex cord stromal tumor. If it is sex cord tumor, what type? granulosa tumor vs sertoli cell tumor.
2. Carcinoid tumor.
3. Primary epithelial tumor. What type? Serous, endometrioid
4. Metastatic tumor. From where?
I think this is good DDX. We must work with IHC stains. I have full panel of IHC results and will paste here next week.
-
本帖最后由 于 2009-08-28 01:32:00 编辑
Now we have our DDX. I can tell you this is an ovarian primary tumor,not metastatic one. Also this is one tumor with different growth patterns, not a mixed tumor.
1. Before I show the IHC results, please give one guess diagnosis, only one dx. What dianosis do you favor based on the H&E slide?
2. What IHC stains do you want to order to distinguish sex cord tumor from carcinoid and epithelial tumor?
Thanks,
cz
As above mentioned, granulosa cell tumors (GCT) have many growth patterns, frequently mixed. CALL-EXNAL小体 often is present in microfollicular pattern. In fact follicular patterns (micro or macro-) have been emphasized in the literature, but are less common than ther others in aggregates.
What percentage of GCTs have CALL-EXNAL小体. I cannot find the answer. Do Some ones can find the percentage with reference? Please paste here. Thank, cz.
By the way I do not meam this case is a GCT or not
| 以下是引用quhong在2009-8-27 21:15:00的发言: This tumor demonstrates variety of patterns, including overt glandular differentiation (figure 1) and trabecular pattern (figure 4). The figure 2 and 3 are difficult to define. I favor cribriform pattern over microfollicular pattern. I do not see typical Call-Exner bodies (follicular structures composed of granulosa cells arranged around a central eosinophilic hyaline or fibrillar material) and coffee beans (cells with longitudinal nuclear groove). Granulosa cell tumor is not favored, but cannot be ruled out. Carcinoid tumor and Sertoli cell tumor should also be in differential diangosis. Metastatic tumor cannot be excluded, either. This is a difficult case. It is really above my pay grade. |
| 以下是引用Liu_Aijun在2009-8-27 8:52:00的发言:
左上图:明确的腺管样结构:支持细胞瘤?类癌?腺癌? 右上图和左下图:同一形态不同倍数,腺管样或CE小体结构:支持细胞瘤?粒层细胞瘤?腺癌? 右下图:梁索结构:支持细胞瘤?粒层细胞瘤?类癌? 综合考虑:1,性索间质肿瘤:(1)支持细胞瘤,(2)两性母细胞瘤,(3)不考虑单纯的粒层细胞瘤。 2,支持细胞瘤与类癌混合性肿瘤。 3,最后需排除宫内膜样腺癌(整体感觉不象腺癌)。 建议标记物:inhibin, EMA, syn,CgA |
Thank Dr. Liu's good analysis. We all should learn from Dr. Liu.
It is correct way to analyze a case-morphology observation, differential diagnosis, immunostains.
For these kinds of cases if you only give one diagnosis even right answer, still it is not correct way.
-
Sertoli cell tumors of the ovary: a clinicopathologic and immunohistochemical study of 54 cases.
James Wright Pathology Laboratories, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA. eoliva@partners.org
Ovarian Sertoli cell tumors are rare, and their morphologic spectrum, behavior, and factors influencing the latter are not clearly established. They may be mimicked by many different tumors, some of them more frequent than Sertoli cell tumors; immunohistochemistry may aid in this differential, but its role has not been analyzed in a large series. We studied the clinicopathologic features of 54 Sertoli cell tumors, including the immunohistochemical profile of 23 of them. The patients, 6 of whom had Peutz-Jeghers syndrome, ranged from 2 to 76 years of age (mean, 30 years). Eleven patients had estrogenic and 4 had androgenic manifestations. The tumors ranged from 0.8 to 30 cm, with the majority being in the range of 4 to 12 cm. They were all unilateral, usually solid, and often yellow. The predominant microscopic pattern was tubular, seen, albeit often only focally, in all tumors; other patterns were cords or trabeculae (28), diffuse (21), pseudopapillary (4), retiform (3), islands or alveolar arrangements (3), and spindled (3). The tubules were solid or hollow with the former being somewhat more common. Delicate septa were occasionally seen and were conspicuous in areas of one tumor. The stroma was abundant in 15 tumors with marked sclerosis in 4. The cells usually had pale to occasionally densely eosinophilic cytoplasm, but 6 tumors were composed of cells with prominent foamy cytoplasm, falling in the category of "lipid-rich" Sertoli cell tumor, and one had cells with clear non-foamy cytoplasm. Forty-four tumors were stage I (42 of them were stage Ia and 2 were stage Ic), 1 was stage II, 3 were stage III, and 6 were not adequately staged. Follow-up was available for 27 patients with stage I tumors, and all were alive and well at last follow-up except for 2 patients with stage Ia and 1 with stage Ic disease. Those 3 patients had pelvic-abdominal recurrences 18, 36, and 9 months, respectively, after the initial diagnosis. Two of the three clinically malignant stage I tumors had moderate to severe cytologic atypia and brisk mitotic activity (>5 or more mitoses/10 high power fields [HPFs]), and one of these had tumor cell necrosis. Among the 10 clinically benign stage I tumors with more than 5 years of follow-up, only 3 had >5 mitoses/10 HPFs, but none had more than mild cytologic atypia and none had tumor cell necrosis. Two of the three patients with stage III disease had follow-up information and one was alive at 16 months and the second developed splenic metastases 2 years after the initial diagnosis. Two of the three stage III tumors had at least moderate cytologic atypia and brisk mitotic activity. Immunohistochemical stains showed positivity for AE1/3-Cam5.2 in 15 of 23 tumors; Epithelial membrane antigen (EMA) was negative in all the tumors. Inhibin was positive in 18 of 22 tumors, calretinin in 10 of 20, CD99 in 19 of 22, vimentin in 17 of 18, smooth muscle actin in 4 of 18, neuron specific enolase in 8 of 16, S-100 in 2 of 20, and chromogranin was negative in all 21 cases studied. Although Sertoli cell tumors usually have a distinctive tubular pattern that facilitates the diagnosis, other patterns may occasionally predominate, causing confusion with various other primary and metastatic ovarian tumors. EMA, inhibin, and chromogranin represent the most helpful triad of immunomarkers serving to exclude two common mimics of Sertoli cell tumors (endometrioid carcinoma [inhibin-; EMA+; chromogranin-] and carcinoid tumor [inhibin-; EMA+; chromogranin+]). Although CD99 and calretinin are often expressed in these tumors, they are much less specific and not as helpful in the differential diagnosis. Most Sertoli cell tumors are stage I, unilateral, cytologically bland, and clinically benign, but occasional examples are high stage, and about 11% of stage I tumors have worrisome histologic features that may portend an adverse outcome. The tumors typically occur in young females, sometimes children who typically present with sexual precocity, and occasional patients have Peutz-Jeghers syndrome.
In term of morphologic study, this is the best and largest paper to describe ov sertoli cell tumor. If you want to know the morphologic features well, please read the original paper. I remeber that I once pasted this abstract some where in the web.
Am J Surg Pathol. 2005 Feb;29(2):143-56.