| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- 子宫肌壁肿块(已补充新图片!!) 请大家发言
-
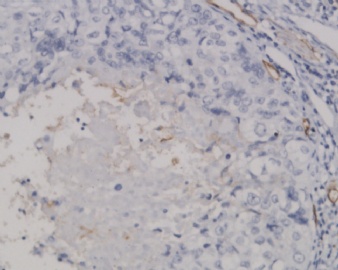
Based on immunohistochemical profile provided by ipath, SCC is out of the picture here and ETT is the most suspicious candidate now. Morphologically I favor it as ETT. If you really want to exclude PSTT, you can add p63 (positive in ETT in about 80% cases, but negative in 100% PSTT) and Mel-CAM (positive in most of PSTT and very rarely positive in ETT) to settle the case. Please keep us updated. Thanks

- 不坠青云之志,长怀赤子之心
Epithelioid Trophoblastic Tumor: Review of a Rare Neoplasm of the Chorionic-Type Intermediate Trophoblast
|
From the Division of Anatomic Pathology, University of Washington Medical Center, Seattle |
We present a brief review of epithelioid trophoblastic tumor, a rare trophoblastic neoplasm derived from chorionic-type intermediate trophoblastic cells that typically presents in reproductive-age women between 1 and 18 years following a previous gestation. Histologic features include a nodular growth pattern of monomorphic, epithelioid cells within a hyaline matrix. Areas of necrosis and mitotic activity (0–9 mitoses per 10 high-power fields) are additional features of this neoplasm. Positive immunostaining for p63 and cytokeratin, frequent location in the lower uterine segment and endocervix, as well as the epithelioid appearance can lead to confusion with squamous cell carcinoma. Inhibin-α is typically expressed, as well as focal, more variable expression of other trophoblastic markers including β-human chorionic gonadotropin, human placental lactogen, placental alkaline phosphate, and Mel-CAM (CD148). The clinical behavior of this rare form of gestational trophoblastic disease is difficult to predict. Although most cases follow a benign course following resection, there is a potential for metastatic disease.
![]() Epithelioid trophoblastic tumor (ETT) was first characterized in 1998 by Drs. Shih and Kurman,1 who recognized it as a rare form of trophoblastic disease composed of intermediate trophoblast cells with features that made it distinct from placental-site trophoblastic tumor and choriocarcinoma. The intermediate trophoblast is thought to have a phenotype that is between that of the primitive cytotrophoblast and the terminally differentiated syncytiotrophoblast. The intermediate trophoblast is the primary cell type in exaggerated placental site, placental site nodule, placental site trophoblastic tumor, and ETT. Based on anatomic location in the pregnant uterus, intermediate trophoblasts can be divided into villous, implantation-site, and chorionic subtypes. The placental chorionic-type intermediate trophoblast has a nodular growth pattern and is thought to be the cell type of origin for lesions with a similar growth pattern, including placental site nodule and ETT. In contrast, placental implantation site intermediate trophoblast tends to have an infiltrative growth pattern, as does its neoplastic counterpart, placental site trophoblastic tumor. Epithelioid trophoblastic tumors are considered a neoplasm composed of chorionic-type intermediate trophoblasts based on histologic characteristics, immunohistochemical expression, and polymerase chain reaction analysis.1–3 Epithelioid trophoblastic tumor can be confused with squamous cell carcinoma because of its frequent involvement of the lower uterine segment or endocervix, its epithelioid histologic appearance, and expression of p63 and cytokeratins. It is important to recognize the trophoblastic nature of this neoplasm and to be able to distinguish it from other neoplastic forms of gestational trophoblastic disease.
Epithelioid trophoblastic tumor (ETT) was first characterized in 1998 by Drs. Shih and Kurman,1 who recognized it as a rare form of trophoblastic disease composed of intermediate trophoblast cells with features that made it distinct from placental-site trophoblastic tumor and choriocarcinoma. The intermediate trophoblast is thought to have a phenotype that is between that of the primitive cytotrophoblast and the terminally differentiated syncytiotrophoblast. The intermediate trophoblast is the primary cell type in exaggerated placental site, placental site nodule, placental site trophoblastic tumor, and ETT. Based on anatomic location in the pregnant uterus, intermediate trophoblasts can be divided into villous, implantation-site, and chorionic subtypes. The placental chorionic-type intermediate trophoblast has a nodular growth pattern and is thought to be the cell type of origin for lesions with a similar growth pattern, including placental site nodule and ETT. In contrast, placental implantation site intermediate trophoblast tends to have an infiltrative growth pattern, as does its neoplastic counterpart, placental site trophoblastic tumor. Epithelioid trophoblastic tumors are considered a neoplasm composed of chorionic-type intermediate trophoblasts based on histologic characteristics, immunohistochemical expression, and polymerase chain reaction analysis.1–3 Epithelioid trophoblastic tumor can be confused with squamous cell carcinoma because of its frequent involvement of the lower uterine segment or endocervix, its epithelioid histologic appearance, and expression of p63 and cytokeratins. It is important to recognize the trophoblastic nature of this neoplasm and to be able to distinguish it from other neoplastic forms of gestational trophoblastic disease.
![]() Clinically, ETT primarily occurs in reproductive-age women following a prior gestation. The time from gestation to presentation varies, with reported intervals ranging from 1 year to as long as 18 years.1,2 A case of ETT has also been reported in a postmenopausal woman.4 Approximately one third of cases arise following a spontaneous abortion or hydatidiform mole, and the majority occur after a full-term pregnancy.1,2 The most common presenting symptom is vaginal bleeding. At the time of diagnosis, serum β-human chorionic gonadotropin levels are usually elevated, but in contrast to choriocarcinoma, levels generally do not exceed 2500 mIU/mL. Prehysterectomy clinical diagnoses range from leiomyomas to carcinomas, and the diagnosis of gestational trophoblastic disease often is not suspected. Although most cases occur in the uterus and endocervix, there are reports of ETT arising in the broad ligament and fallopian tube.5,6
Clinically, ETT primarily occurs in reproductive-age women following a prior gestation. The time from gestation to presentation varies, with reported intervals ranging from 1 year to as long as 18 years.1,2 A case of ETT has also been reported in a postmenopausal woman.4 Approximately one third of cases arise following a spontaneous abortion or hydatidiform mole, and the majority occur after a full-term pregnancy.1,2 The most common presenting symptom is vaginal bleeding. At the time of diagnosis, serum β-human chorionic gonadotropin levels are usually elevated, but in contrast to choriocarcinoma, levels generally do not exceed 2500 mIU/mL. Prehysterectomy clinical diagnoses range from leiomyomas to carcinomas, and the diagnosis of gestational trophoblastic disease often is not suspected. Although most cases occur in the uterus and endocervix, there are reports of ETT arising in the broad ligament and fallopian tube.5,6
![]() Grossly, ETT forms a well-defined, expansile mass in the uterine wall, lower uterine segment, or endocervix. It has a solid to cystic, fleshy appearance, and can have variable degrees of hemorrhage and necrosis on cut sections. Gross size generally ranges between 2 and 5 centimeters.
Grossly, ETT forms a well-defined, expansile mass in the uterine wall, lower uterine segment, or endocervix. It has a solid to cystic, fleshy appearance, and can have variable degrees of hemorrhage and necrosis on cut sections. Gross size generally ranges between 2 and 5 centimeters.
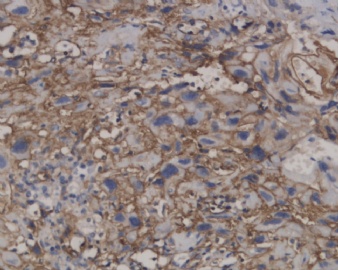
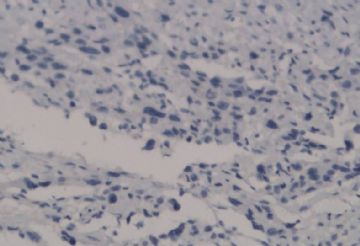
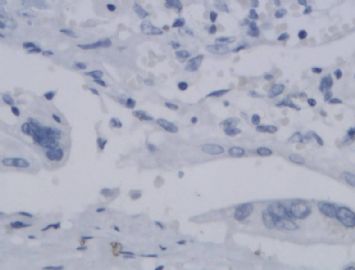
![]() Microscopically, the growth pattern of ETT is nodular, with nests and cords of monomorphic cells with eosinophilic to clear cytoplasm set in a classically hyaline matrix. It has generally well-circumscribed borders, but it is not uncommon to find some focal infiltrative peripheral areas. Although the predominant cell type is the small, epithelioid, chorionic-type intermediate trophoblast, occasional syncytiotrophoblastic cells may be present as well; unless they represent a substantial population, they do not warrant a diagnosis of choriocarcinoma. Necrosis can be prominent and apoptotic cells are frequent. Figure 1A onclick="ViewImage('/arpaonline/?request=display-figures\x26name=i1543-2165-130-12-1875-f01'); return false;" href="http://arpa.allenpress.com/arpaonline/?request=display-figures&name=i1543-2165-130-12-1875-f01" target="_blank">
Microscopically, the growth pattern of ETT is nodular, with nests and cords of monomorphic cells with eosinophilic to clear cytoplasm set in a classically hyaline matrix. It has generally well-circumscribed borders, but it is not uncommon to find some focal infiltrative peripheral areas. Although the predominant cell type is the small, epithelioid, chorionic-type intermediate trophoblast, occasional syncytiotrophoblastic cells may be present as well; unless they represent a substantial population, they do not warrant a diagnosis of choriocarcinoma. Necrosis can be prominent and apoptotic cells are frequent. Figure 1A onclick="ViewImage('/arpaonline/?request=display-figures\x26name=i1543-2165-130-12-1875-f01'); return false;" href="http://arpa.allenpress.com/arpaonline/?request=display-figures&name=i1543-2165-130-12-1875-f01" target="_blank"> shows a case with prominent regions of geographic necrosis surrounded by islands of viable neoplastic cells. The neoplastic cells are small and epithelioid with eosinophilic cytoplasm, and are embedded in a hyaline matrix (Figure 2B onclick="ViewImage('/arpaonline/?request=display-figures\x26name=i1543-2165-130-12-1875-f01'); return false;" href="http://arpa.allenpress.com/arpaonline/?request=display-figures&name=i1543-2165-130-12-1875-f01" target="_blank">
shows a case with prominent regions of geographic necrosis surrounded by islands of viable neoplastic cells. The neoplastic cells are small and epithelioid with eosinophilic cytoplasm, and are embedded in a hyaline matrix (Figure 2B onclick="ViewImage('/arpaonline/?request=display-figures\x26name=i1543-2165-130-12-1875-f01'); return false;" href="http://arpa.allenpress.com/arpaonline/?request=display-figures&name=i1543-2165-130-12-1875-f01" target="_blank"> ). Decidualized stromal cells can also be a useful diagnostic clue in biopsy specimens when the differential diagnosis includes squamous carcinoma. Mitoses in ETT are reported to range from 0 to 9 per 10 high-power fields (mean of 2/10) and the Ki-67/MIB1 proliferative index ranges from 10% to 25%.1,2
). Decidualized stromal cells can also be a useful diagnostic clue in biopsy specimens when the differential diagnosis includes squamous carcinoma. Mitoses in ETT are reported to range from 0 to 9 per 10 high-power fields (mean of 2/10) and the Ki-67/MIB1 proliferative index ranges from 10% to 25%.1,2
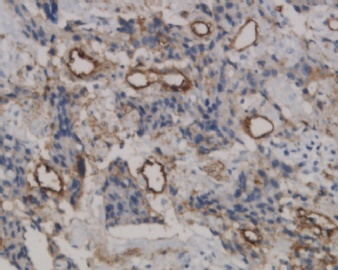
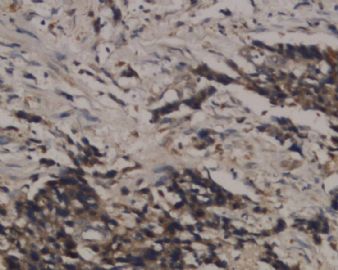
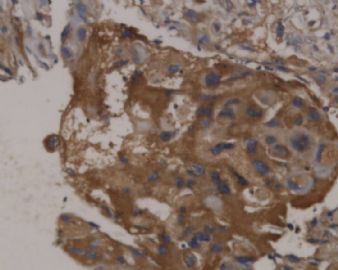
![]() Epithelioid trophoblastic tumor has immunohistochemical expression of markers seen in normal chorionic-type intermediate trophoblasts, including diffuse expression of pancytokeratin, cytokeratin 18, epithelial membrane antigen, and, interestingly, p63.7 Most cells also express inhibin-α. Other trophoblastic markers including β-human chorionic gonadotropin, human placental lactogen, placental alkaline phosphate, and Mel-CAM (CD146) are only focally expressed. Figures 1C and 1D onclick="ViewImage('/arpaonline/?request=display-figures\x26name=i1543-2165-130-12-1875-f01'); return false;" href="http://arpa.allenpress.com/arpaonline/?request=display-figures&name=i1543-2165-130-12-1875-f01" target="_blank">
Epithelioid trophoblastic tumor has immunohistochemical expression of markers seen in normal chorionic-type intermediate trophoblasts, including diffuse expression of pancytokeratin, cytokeratin 18, epithelial membrane antigen, and, interestingly, p63.7 Most cells also express inhibin-α. Other trophoblastic markers including β-human chorionic gonadotropin, human placental lactogen, placental alkaline phosphate, and Mel-CAM (CD146) are only focally expressed. Figures 1C and 1D onclick="ViewImage('/arpaonline/?request=display-figures\x26name=i1543-2165-130-12-1875-f01'); return false;" href="http://arpa.allenpress.com/arpaonline/?request=display-figures&name=i1543-2165-130-12-1875-f01" target="_blank"> show an example of an ETT with strong and diffuse expression of cytokeratin 7 and p63, respectively.
show an example of an ETT with strong and diffuse expression of cytokeratin 7 and p63, respectively.
![]() Epithelioid trophoblastic tumor can be confused with squamous cell carcinoma because of its epithelioid appearance and frequent location in the lower uterine segment. The hyaline matrix and necrosis can resemble keratin, and ETT can grow along the surface of the cervix, similar to cervical squamous cell carcinoma. Several cases have been reported that were initially misdiagnosed as cervical squamous carcinomas because of in situ cervical spread.8,9 Extrauterine ETT can be an especially difficult diagnosis if it presents as a metastatic lesion.10 In addition, p63 and cytokeratin expression can misleadingly support a diagnosis of squamous cell carcinoma. Trophoblastic markers should be employed in an unusual uterine epithelioid neoplasm to help in this key differential diagnosis. Inhibin-α and cytokeratin 18 should be expressed in ETT and not in squamous cell carcinoma. Other clues favoring a diagnosis of ETT instead of squamous cell carcinoma include the presence of associated decidualized stromal cells and the absence of intercellular bridges between the neoplastic cells. Clinically, β-human chorionic gonadotropin levels and history of human papillomavirus infection can also be helpful, although they can be misleading. Epithelioid smooth muscle tumors may also be in the differential diagnosis of a mass found in the endomyometrium composed of epithelioid cells. However, smooth muscle tumors can be excluded from the differential diagnosis by the lack of expression of muscle markers, desmin, and smooth muscle actin in ETTs.
Epithelioid trophoblastic tumor can be confused with squamous cell carcinoma because of its epithelioid appearance and frequent location in the lower uterine segment. The hyaline matrix and necrosis can resemble keratin, and ETT can grow along the surface of the cervix, similar to cervical squamous cell carcinoma. Several cases have been reported that were initially misdiagnosed as cervical squamous carcinomas because of in situ cervical spread.8,9 Extrauterine ETT can be an especially difficult diagnosis if it presents as a metastatic lesion.10 In addition, p63 and cytokeratin expression can misleadingly support a diagnosis of squamous cell carcinoma. Trophoblastic markers should be employed in an unusual uterine epithelioid neoplasm to help in this key differential diagnosis. Inhibin-α and cytokeratin 18 should be expressed in ETT and not in squamous cell carcinoma. Other clues favoring a diagnosis of ETT instead of squamous cell carcinoma include the presence of associated decidualized stromal cells and the absence of intercellular bridges between the neoplastic cells. Clinically, β-human chorionic gonadotropin levels and history of human papillomavirus infection can also be helpful, although they can be misleading. Epithelioid smooth muscle tumors may also be in the differential diagnosis of a mass found in the endomyometrium composed of epithelioid cells. However, smooth muscle tumors can be excluded from the differential diagnosis by the lack of expression of muscle markers, desmin, and smooth muscle actin in ETTs.
![]() After recognizing the trophoblastic differentiation of the neoplasm, ETT should be distinguished from other forms of trophoblastic disease. Placental site nodule, also thought to originate from chorionic-type intermediate trophoblasts, understandably shares some histologic and immunohistochemical features with ETT. Placental site nodule and ETT have similar nodular growth patterns, hyaline stroma, and expansile borders. However, placental site nodules are smaller, less cellular, do not contain necrosis, and have a Ki-67 proliferative index generally below 10%. In addition, placental site nodules are usually an incidental finding, and ETTs generally form a clinically recognized mass. It is theorized that ETTs are the neoplastic counterpart of placental site nodule, so a spectrum between these 2 entities may exist.
After recognizing the trophoblastic differentiation of the neoplasm, ETT should be distinguished from other forms of trophoblastic disease. Placental site nodule, also thought to originate from chorionic-type intermediate trophoblasts, understandably shares some histologic and immunohistochemical features with ETT. Placental site nodule and ETT have similar nodular growth patterns, hyaline stroma, and expansile borders. However, placental site nodules are smaller, less cellular, do not contain necrosis, and have a Ki-67 proliferative index generally below 10%. In addition, placental site nodules are usually an incidental finding, and ETTs generally form a clinically recognized mass. It is theorized that ETTs are the neoplastic counterpart of placental site nodule, so a spectrum between these 2 entities may exist.
![]() Placental site trophoblastic tumor is differentiated from ETT by its very infiltrative growth pattern, prominent vascular invasion, and slightly larger implantation site-type intermediate trophoblast cells. In addition, placental site trophoblastic tumor will be more diffusely positive for human placental lactogen and Mel-CAM and will lack p63 expression. Choriocarcinoma is distinguished by its dimorphic population with larger areas of β-human chorionic gonadotropin–positive synciotrophoblast cells. However, it should be recognized that there are several reports of cases diagnosed as ETT coexisting with choriocarcinoma.11,12 A summary of useful immunohistochemical features of ETT and other entities in the differential diagnosis is presented in the Table onclick="ViewImage('/arpaonline/?request=display-figures\x26name=i1543-2165-130-12-1875-t01'); return false;" href="http://arpa.allenpress.com/arpaonline/?request=display-figures&name=i1543-2165-130-12-1875-t01" target="_blank">
Placental site trophoblastic tumor is differentiated from ETT by its very infiltrative growth pattern, prominent vascular invasion, and slightly larger implantation site-type intermediate trophoblast cells. In addition, placental site trophoblastic tumor will be more diffusely positive for human placental lactogen and Mel-CAM and will lack p63 expression. Choriocarcinoma is distinguished by its dimorphic population with larger areas of β-human chorionic gonadotropin–positive synciotrophoblast cells. However, it should be recognized that there are several reports of cases diagnosed as ETT coexisting with choriocarcinoma.11,12 A summary of useful immunohistochemical features of ETT and other entities in the differential diagnosis is presented in the Table onclick="ViewImage('/arpaonline/?request=display-figures\x26name=i1543-2165-130-12-1875-t01'); return false;" href="http://arpa.allenpress.com/arpaonline/?request=display-figures&name=i1543-2165-130-12-1875-t01" target="_blank"> .
.
![]() Because of the rarity of the neoplasm, the biologic behavior of ETTs has not been firmly established, but the metastatic potential appears to be similar to placental site trophoblastic tumor. Shih and Kurman,1,2 in their seminal series, reported a metatstatic rate of 25% and a mortality rate of 10%. Although histologic features to predict outcome are not well established, some have suggested an association between high mitotic index and more aggressive behavior. In a recent series of 5 cases of ETT reported by Fadare et al,8 the only patient who died of disease had a mitotic rate of 48 mitoses per 10 high-power fields. There is also a suggestion that ETT may not be as chemosensitive as other gestational trophoblastic diseases.1 Control and cure of localized disease has been achieved with hysterectomy in most cases.
Because of the rarity of the neoplasm, the biologic behavior of ETTs has not been firmly established, but the metastatic potential appears to be similar to placental site trophoblastic tumor. Shih and Kurman,1,2 in their seminal series, reported a metatstatic rate of 25% and a mortality rate of 10%. Although histologic features to predict outcome are not well established, some have suggested an association between high mitotic index and more aggressive behavior. In a recent series of 5 cases of ETT reported by Fadare et al,8 the only patient who died of disease had a mitotic rate of 48 mitoses per 10 high-power fields. There is also a suggestion that ETT may not be as chemosensitive as other gestational trophoblastic diseases.1 Control and cure of localized disease has been achieved with hysterectomy in most cases.
![]() In summary, ETT is a neoplasm of the chorionic-type intermediate trophoblast with an unpredictable but usually indolent behavior. It is important to distinguish ETT from squamous cell carcinoma, as well as other trophoblastic neoplasms. Immunohistochemistry and clinical presentation can be essential in working through this differential diagnosis.
In summary, ETT is a neoplasm of the chorionic-type intermediate trophoblast with an unpredictable but usually indolent behavior. It is important to distinguish ETT from squamous cell carcinoma, as well as other trophoblastic neoplasms. Immunohistochemistry and clinical presentation can be essential in working through this differential diagnosis.
2. Shih IM, Kurman RJ. The pathology of intermediate trophoblastic tumors and tumor-like lesions. Int J Gynecol Pathol 2001;20:31–47. [PubMed Citation]
3. Oldt RJ, Kurman RJ, Shih IM. Molecular genetic analysis of placental site trophoblastic tumors and epithelioid trophoblastic tumors confirms their trophoblastic origin. Am J Pathol 2002;161:1033–1037. [PubMed Citation]
4. Coulson LE, Kong CS, Zaloudek C. Epithelioid trophoblastic tumor of the uterus in a postmenopausal woman: a case report and review of the literature. Am J Surg Pathol 2000;24:1558–1562. [PubMed Citation]
5. Kuo Kt, Chen MJ, Lin MC. Epithelioid trophoblastic tumor of the broad ligament: a case report and review of the literature. Am J Surg Pathol 2004;28:405–409. [PubMed Citation]
6. Parker A, Lee V, Dalrymple C. et al. Epithelioid trophoblastic tumor: report of a case in the fallopian tube. Pathology 2003;35:136–140. [PubMed Citation]
7. Shih IM, Kurman RJ. P63 expression is useful in the distinction of epithelioid trophoblastic and placental site trophoblastic tumors by profiling trophoblastic subpopulations. Am J Surg Pathol 2004;28:1177–1183. [PubMed Citation]
8. Fadare O, Parkash V, Carcangiu ML, Hui P. Epithelioid trophoblastic tumor: clinicopathologic features with an emphasis on uterine cervical involvement. Mod Pathol 2006;19:74–72.
9. Narita F, Takeuchi K, Haman S. et al. Epithelioid trophoblastic tumor (ETT) initially interpreted as cervical cancer. Int J Gynecol Caner 2003;13:551–554. [PubMed Citation]
10. Hamazaki S, Nakamoto S, Okino T. et al. Epithelioid trophoblastic tumor: morphologic and immunohistochemical study of three lung lesions. Hum Pathol 1999;30:1321–1327. [PubMed Citation]
11. Shen DHS, Khoo US, Ngan HYS. et al. Coexisting epithelioid trophoblastic tumor and choriocarcinoma of the uterus following a chemoresistant hydatidiform mole. Arch Pathol Lab Med 2003;127:e291–e293.
12. Knox S, Brooks SE, Wong-You-Cheong J. et al. Choriocarcinoma and epithelial trophoblastic tumor: successful treatment of relapse with a hysterectomy and high-dose chemotherapy with peripheral stem cell support: a case report. Gynecol Oncol 2002;85:204–208. [PubMed Citation]
![]() Immunohistochemical Expression Useful in the Differential Diagnosis of Epithelioid Trophoblastic Tumors
Immunohistochemical Expression Useful in the Differential Diagnosis of Epithelioid Trophoblastic Tumors


Click on thumbnail for full-sized image.
![]() Histologic and immunohistochemical features of epithelioid trophoblastic tumor (ETT). A, An ETT with an area of necrosis surrounded by a circumscribed rim of neoplastic epithelioid cells growing in nests and cords (hematoxylin-eosin, original magnification ×40). B, The chorionic-type intermediate trophoblastic cells that make up ETT are small and epithelioid with eosinophilic cytoplasm, and are set in a distinct hyaline matrix. Mitotic activity can be notable but generally is below 9 mitoses per 10 high-power fields. Also note the decidualized cells in the surrounding endometrial stroma (hematoxylin-eosin, original magnification ×600). C, Similar to other forms of gestational trophoblastic disease, ETT expresses cytokeratins, including cytokeratin 7, pictured here (original magnification ×100). D, One of the features distinguishing ETT from placental site trophoblastic tumor is nuclear p63 expression, as shown here. However, p63 and cytokeratin coexpression can lead to a mistaken diagnosis of carcinoma with a squamous or transitional phenotype (original magnification ×400)
Histologic and immunohistochemical features of epithelioid trophoblastic tumor (ETT). A, An ETT with an area of necrosis surrounded by a circumscribed rim of neoplastic epithelioid cells growing in nests and cords (hematoxylin-eosin, original magnification ×40). B, The chorionic-type intermediate trophoblastic cells that make up ETT are small and epithelioid with eosinophilic cytoplasm, and are set in a distinct hyaline matrix. Mitotic activity can be notable but generally is below 9 mitoses per 10 high-power fields. Also note the decidualized cells in the surrounding endometrial stroma (hematoxylin-eosin, original magnification ×600). C, Similar to other forms of gestational trophoblastic disease, ETT expresses cytokeratins, including cytokeratin 7, pictured here (original magnification ×100). D, One of the features distinguishing ETT from placental site trophoblastic tumor is nuclear p63 expression, as shown here. However, p63 and cytokeratin coexpression can lead to a mistaken diagnosis of carcinoma with a squamous or transitional phenotype (original magnification ×400)
As Dr. Yang mentioned you should add a p63 stain for this case.
My feeling is IHC stains are very important for dx of these trophoblastic tumors. PSTT and ETT share similar morphology (though we can describe many different morphologic features) and prognosis. ETT was first characterized in 1998 by Drs. Shih and Kurman fro John Hopkins. In now days pathologists make the classification of these tumore more and more complicated. Dr. Silva from MD Anderson once said that he did not think it was clinical useful to separate ETT from PSTT. However we have to separate them now.


 )
)