| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
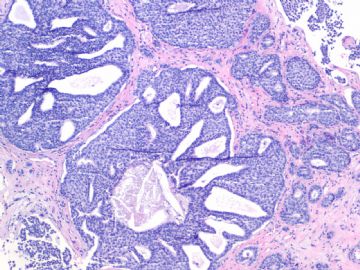
- B1757Invasive ductal ca look as DCIS-the importance of myoepithelial marker (cqz-10)
| 姓 名: | ××× | 性别: | 年龄: | ||
| 标本名称: | |||||
| 简要病史: | |||||
| 肉眼检查: | |||||
We are in 2009 already. I send this easy case for you.
Breast core biopsy:
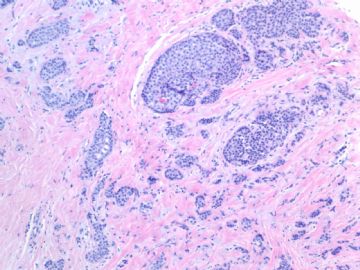
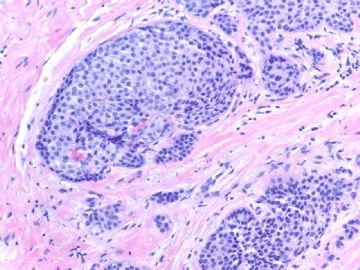
Fig 1, 2: one area 100x, 200x
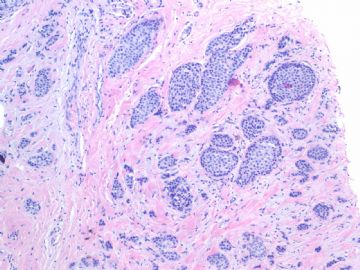
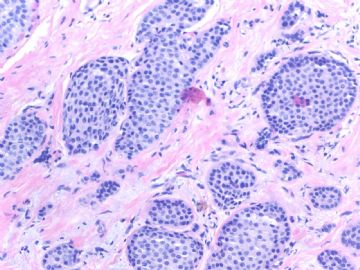
Fig 3, 4: the second area 100x, 200x
Fig 5: the third area 100x
What is your interpretation?
-
本帖最后由 于 2009-07-19 05:14:00 编辑
相关帖子
- • 乳腺肿物
- • 乳腺肿物
- • 乳腺癌吗??
- • 左乳肿块,协助诊断
- • 乳腺肿物
- • 乳腺小管癌?
- • 左乳肿瘤--浸润性导管癌?
- • 看看这是那个类型的乳腺癌?
- • 乳腺肿物,请大家帮忙会诊是恶性的吗??
- • 急`1`1`1`1乳腺肿物,请大家帮忙会诊
-
本帖最后由 于 2009-02-28 12:11:00 编辑
What lesson do we learn from this case?
1. Large tumor nests can be invasive carcinoma. In other words the invasive ca can look like DCIS. This is very important in the breat core bx.
2. If you are not sure, please always do the myoepithelial stains.
3. The stains of some markers can be reduced or negative in DCIS. It is better to do two markers together. We often use p63 and SMMHC.
4. SMA is most sensitive marker. However its specificity is low. Sometimes it is difficult to interpretate. See above phoots. We seldom use it.
Ok, this it. Thanks, cz
I paste here abstract, a very good new paper about myoepithelial cells from Dr Schnitt Harvard group. If you are breast pathologists you should know Dr. Schnitt who is an excellent breast pathologist. This paper was just published in Feb. 2009 in American Journal of surgical Pathology (Am J Surg Path). Am J Surg Path is the best surgical pathology journal.
Hilson JB, Schnitt SJ, Collins LC.
Phenotypic alterations in ductal carcinoma in situ-associated myoepithelial cells: biologic and diagnostic implications.
Am J Surg Pathol. 2009 Feb;33(2):227-32.
Department of Pathology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215, USA.
Recent molecular studies have indicated that ductal carcinoma in situ (DCIS)-associated myoepithelial cells (MECs) show differences from MECs in normal breast tissue. Such alterations may influence the progression of DCIS to invasive cancer. The purpose of this study was to investigate further phenotypic alterations in DCIS-associated MECs. Paraffin sections of 101 cases of DCIS (56 without and 45 with associated invasive carcinoma) were immunostained for 7 MEC markers: smooth muscle actin, smooth muscle myosin heavy chain (SMMHC), calponin, p63, cytokeratin (CK) 5/6, CD10, and p75. In each case, the distribution and intensity of staining for each marker in DCIS-associated MECs was compared with that in MECs surrounding normal ductal-lobular structures on the same slide. In 85 cases (84.2%), DCIS-associated MECs showed decreased expression of one or more MEC markers when compared with normal MECs. The proportion of cases that showed reduced expression was 76.5% for SMMHC, 34.0% for CD10, 30.2% for CK5/6, 17.4% for calponin, 12.6% for p63, 4.2% for p75, and 1% for smooth muscle actin. Reduced MEC expression of SMMHC was significantly more frequent in high grade than in non-high-grade DCIS (84.8% vs. 61.5% of cases, P=0.01). We conclude that DCIS-associated MECs show immunophenotypic differences from MECs surrounding normal mammary ductal-lobular structures. The biologic significance of this remains to be determined. However, these results indicate that the sensitivity of some MEC markers is lower in DCIS-associated MECs than in normal MECs. This observation should be taken into consideration when selecting MEC markers to help distinguish in situ from invasive breast carcinomas.