| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- B4323也许是少见病例,供大家一起学习!
| 性别 | 女 | 年龄 | 39岁 | 临床诊断 | 盆腔肿物 |
|---|---|---|---|---|---|
| 一般病史 | 2016年3月份发现卵巢肿物约卵黄大小。术前超声:盆腔可见非均质包块,约112×96.9×75.5mm,内回声杂乱。 | ||||
| 标本名称 | 左卵巢囊肿 | ||||
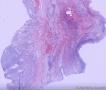
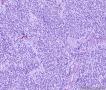
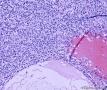
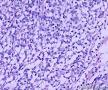
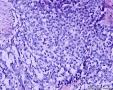
| 大体所见 | (左卵巢囊肿)不整形组织2块,总大小约10.0×9.0×7.0cm。大块组织表面光滑覆浆膜,附输卵管组织,长7.0cm,最大径1.0cm,伞端可见。部分呈实性,灰黄灰白色,质中,部分呈囊壁样,内壁暗红毛糙。小块组织表面暗红色,粗糙,切面实性,灰白灰红,可见坏死样物。 | ||||

- 过河小马!
yolk sac tumor
-
wangruihui: 卵巢卵黄囊瘤(内胚窦瘤)。2016-05-21 14:35
-
dama0609: 老师们,能否讲一下诊断依据,鉴别诊断!2016-05-22 08:23

知之者不如好之者,好之者不如乐之者。(语出幽梦影)
各位老师好:
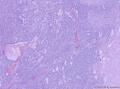
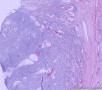
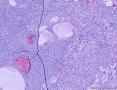
此前,刘老师远程会诊意见:(左卵巢)初步考虑性索间质肿瘤,微囊性间质瘤可能性大。
建议染组化CD10,CD99,a-inhibin,calretinin,CgA,Syn,CD56,SMA,KI67,CKpan,CK7,EMA,WT-1,b-catinin。
我们提的组化今日出的结果:SALL4(-),ER(-),PAX8(-),WT-1(-),P53散在+(野生型),NapsinA(-),inhibin(-),CR(-),CD56(+),CD99(-),CK(-),KI67(40%+)。
组化提于刘老师会诊之前,当初诊断思路不甚正确,所以组化项目组合不太恰当,请各位谅解!
求各位老师您的宝贵意见!谢谢。

- 过河小马!
-
本帖最后由 abin 于 2016-06-28 08:57:06 编辑
形态学考虑性索间质肿瘤(幼年性粒层细胞瘤或微囊性间质瘤),但免疫组化性索标记物仅CD56阳性,不太好解释。

华夏病理/粉蓝医疗
为基层医院病理科提供全面解决方案,
努力让人人享有便捷准确可靠的病理诊断服务。
-
13578675159 离线
- 帖子:1
- 粉蓝豆:49
- 经验:4
- 注册时间:2016-01-12
- 加关注 | 发消息
Am J Surg Pathol 2009 Mar;33 (3):367-75.
Microcystic stromal tumor of the ovary: report of 16 cases of a hitherto uncharacterized distinctive ovarian neoplasm.
Irving JA , Young RH .
Department of Laboratory Medicine, Pathology, and Medical Genetics, Royal Jubilee Hospital, Victoria, BC, Canada. Julie.Irving@viha.ca
Abstract
We have encountered 16 ovarian neoplasms of probable stromal origin whose most distinctive feature is microcystic change, which is usually conspicuous. On the basis of our extensive experience with ovarian tumors, the neoplasm is unique and warrants separate categorization; we have elected to designate it "microcystic stromal tumor" because of its most striking feature. The patients ranged from 26 to 63 (mean 45) years of age and typically presented with a pelvic mass. Hormonal manifestations were possibly present in only 2. All tumors were unilateral with a mean size of 8.7 (range: 2 to 27) cm and none had evidence of extraovarian spread. The tumors were solid-cystic (11 cases), solid (3 cases), or predominantly cystic (2 cases). The solid component was usually firm and tan or white-tan, but in 1 case was yellowish; soft foci were present in 3 cases and small foci of hemorrhage, necrosis, or both, in 3. On microscopic examination the appearance of the tumors varied according to the relative prominence of their 3 fundamental components: microcysts, solid cellular regions, and fibrous stroma. Microcysts dominated in 9 cases, were roughly equal to noncystic morphology in 5 cases and were minor in 2. The microcystic pattern was characterized by small rounded to oval cystic spaces, in areas coalescing to larger irregular channels; intracytoplasmic vacuoles were also frequently present. The solid cellular areas were usually focally intersected by fibrous bands and hyaline plaques reminiscent of thecoma. The cells contained moderately conspicuous finely granular, lightly eosinophilic cytoplasm, with generally bland, round to oval or spindle-shaped nuclei with fine chromatin and small indistinct nucleoli. Foci of bizarre nuclei were, however, present in 10 cases. Mitotic rate was low in all cases, ranging from 0 to 2 mitoses/10 high-power fields. Immunohistochemical results were as follows: CD10, 16/16 cases positive; vimentin, 16/16 cases positive; inhibin, 1/16 cases weakly positive; calretinin, 1/16 cases positive; cytokeratin, 4/16 cases focally positive; and epithelial membrane antigen, 0/16 cases positive. Microcystic change can be observed in a wide variety of ovarian tumors and the broad potential differential diagnosis is discussed in the text. For tumors which have been well sampled and exhibit (1) a microcystic pattern and regions with lobulated cellular masses with intervening, sometimes hyalinized fibrous stroma, (2) an absence of morphologic features enabling any other specific diagnosis in the sex cord-stromal category, (3) an absence of epithelial elements, and (4) an absence of teratomatous or other germ cell elements, we propose the designation "microcystic stromal tumor." The characteristic immunophenotype is CD10/vimentin+/epithelial membrane antigen-, with focal cytokeratin-positivity in one-quarter of cases; inhibin and/or calretinin are usually negative. Seven patients with available follow-up are without evidence of disease at a mean of 4.25 years (range: 1.5 to 12.5 y) from the time of initial diagnosis. These tumors, to date, have occurred over a wide age range in postpubertal females, are characteristically unilateral, and confined to the ovary at presentation. They represent, in addition to the sclerosing stromal tumor (segregated out 3 decades ago), a distinctive subtype of ovarian tumor, likely also belonging to the stromal category based on current evidence.
PMID: 18971779 [ - ]

华夏病理/粉蓝医疗
为基层医院病理科提供全面解决方案,
努力让人人享有便捷准确可靠的病理诊断服务。
Am J Surg Pathol 2015 Oct;39 (10):1420-6.
Microcystic Stromal Tumor: A Distinctive Ovarian Sex Cord-Stromal Neoplasm Characterized by FOXL2, SF-1, WT-1, Cyclin D1, and β-catenin Nuclear Expression and CTNNB1 Mutations.
Irving JA , Lee CH , Yip S , Oliva E , McCluggage WG , Young RH .
Abstract
Since our first description of the microcystic stromal tumor (MST) of the ovary, a rare and distinctive neoplasm with a definitional, usually striking microcystic pattern and a CD10+/vimentin+/inhibin-/calretinin- immunophenotype, 3 examples with β-catenin nuclear localization, and CTNNB1 mutation have been reported. We undertook a detailed immunohistochemical study and molecular analysis of CTNNB1 and FOXL2 of 15 cases of MST to further characterize this neoplasm and establish its histogenesis. Diffuse nuclear staining for FOXL2, WT-1, cyclin D1, and β-catenin was present in all tumors tested, and 12/15 were positive for steroidogenic factor-1 (SF-1). Heterozygous missense point mutations in exon 3 of CTNNB1 were detected in 8 of 14 cases, resulting in amino acid changes at codons 32, 34, 35, and 37. There was no correlation between CTNNB1 exon 3 mutation status and tumor immunophenotype. All 14 cases tested showed wild-type FOXL2. Our study establishes that MST of the ovary exhibits a characteristic FOXL2/SF-1/WT-1/cyclin D1/nuclear β-catenin-positive immunohistochemical profile, which may be useful in diagnosis and in the exclusion of histologic mimics. The presence of diffuse nuclear FOXL2 and WT-1 immunostaining in all cases and SF-1 in most supports the classification of MST within the sex cord-stromal category. Aberrant nuclear β-catenin expression, detected in all MSTs, appears to be the result of stabilizing CTNNB1 mutations in 57% of cases, providing further evidence that dysregulation of the Wnt/B-catenin pathway is involved in the tumorigenesis of MST and may involve activation of β-catenin with upregulation of cyclin D1.
PMID: 26200099 [Pubmed - In-Process]

华夏病理/粉蓝医疗
为基层医院病理科提供全面解决方案,
努力让人人享有便捷准确可靠的病理诊断服务。
-
本帖最后由 abin 于 2016-06-28 08:52:54 编辑
卵巢的微囊性间质瘤:报道16例至今尚未阐明其特征的卵巢肿瘤
摘要:
我们遇到16例可能来源于卵巢间质的肿瘤,它们最显著的特征是微囊改变,微囊通常非常明显。基于我们对(诊断)卵巢肿瘤的丰富经验,这种肿瘤是独特的,有必要单独分类;因其显著的形态学特征而建议命名为“微囊性间质瘤”。患者年龄范围26-63岁(中位年龄45岁),通常表现为盆腔肿块。仅2例可能出现激素紊乱的表现。所有肿瘤均为单侧性,平均大小8.7cm(范围2-27cm),均无卵巢外扩散证据。肿瘤呈囊实性(11例),实性(3例),或囊性为主(2例)。实性区域通常质硬,褐色或者白-褐色,但一例呈黄色。3例切面小灶质软,另有3例见小灶出血和/或坏死。镜下,肿瘤中3种基本部分(微囊,实性富细胞区域,纤维间质)的相对比例有所变化。9例以微囊形态为主,5例微囊与非囊性形态比例相当,2例微囊形态较少。微囊的特征是小圆形或卵圆形空腔,部分区域融合成大的不规则腔隙。胞质内空泡也常见。实性富细胞区常被纤维间质局灶性分隔,并穿插着类似卵泡膜细胞瘤的透明斑块。细胞含有中等量明显的细颗粒性淡嗜酸性胞质。细胞核形态温和,呈圆形至卵圆形或梭形,染色质细腻,核仁小,不明显。但是10例局灶出现奇异形细胞核。所有病例的核分裂指数低,范围0-2个/10HPF。免疫组化:CD10,16/16例阳性;Vim,16/16例阳性;Inhibin,1/16例弱阳性;calretinin,1/16例阳性;CK,4/16例局灶阳性;EMA,0/16例阳性。微囊性改变可见于多种卵巢肿瘤,本文将讨论其广泛的鉴别诊断。充分取材,呈现以下特征的肿瘤:(1)微囊结构和分叶状富细胞区伴穿插的、有时透明变性的纤维间质,(2)形态学特征无法归入性索-间质肿瘤的其他特殊类型,(3)不存在上皮成分,(4)缺少畸胎瘤或其他生殖细胞成分,我们提议命名为“微囊性间质肿瘤”。免疫组化特点:CD10+/Vim+/EMA-,1/4病例呈CK局灶阳性,而抑制素和/或calretinin通常阴性。7名患者有随访资料,初次诊断后平均4.25年(1.5-12.5年)无病生存。到目前为止,这些肿瘤在成年女性中发病年龄较广,单侧性,就诊时局限于卵巢。排除硬化性间质瘤(多数<30岁)之后,这类肿瘤代表一种独特的卵巢肿瘤亚型,基于目前的研究证据,它们可能属于卵巢间质性肿瘤。