| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- B4272孔祥田老师病例讨论:自生免疫性化生性萎缩性胃炎!已点评
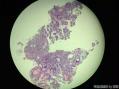
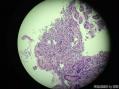
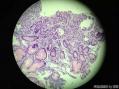
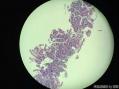
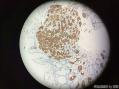
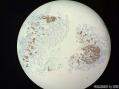
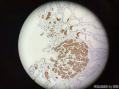
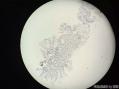
64 yo F gastric body biopsy. Multiple pieces from the gastric body received. Synaptophysin IHC showed here, same pattern on Chromogranin A IHC(did not show here). No parietal cells seen in these biopsies. Intestinal metaplasia and pseudopyloric metaplasia seen. No dysplasia identified. Pictures from the same patient and same location. You can see intestinal metaplasia on pictures 8-9 here. Dx?
-
本帖最后由 芳芸 于 2015-11-20 16:27:09 编辑

让每一家医院都拥有精细化亚专科服务;让人人享有便捷、准确、可靠的病理诊断服务。 疑难会诊咨询热线400-0098-600 柳芳芸 15968153692。
-
本帖最后由 芳芸 于 2015-11-18 15:36:08 编辑
1. 胃,胃体,活组织检查(STOMACH, BODY, BIOPSY)
胃体型黏膜伴高分化神经内分泌肿瘤/类癌,后者发生于肠色素细胞样(enterochromaffin cell-like,ECL)细胞增生背景,通过嗜铬蛋白A(chromogranin A,CgA)和突触素(synaptophysin,Syn)免疫组化染色得以证实。Ki-67增殖指数约为1%。见诊断评论。
萎缩性胃炎伴壁细胞完全性缺失、广泛肠上皮化生和幽门腺化生,形态学上提示为自身免疫性化生性萎缩性胃炎(autoimmune metaplastic atrophic gastritis,AMAG)。见诊断评论及参考文献。
幽门螺杆菌(Helicobacter pylori,HP)Giemsa染色呈阴性。
腺体无异型。
2. 胃,胃窦,活组织检查(STOMACH, ANTRUM,BIOPSY)
胃窦型黏膜伴慢性非活动性胃炎。
幽门螺杆菌(Helicobacter pylori,HP)Giemsa染色和免疫组化染色都呈阴性。
突触素(synaptophysin,Syn)和嗜铬蛋白A(chromogranin A,CgA)免疫组化染色证实G细胞增生。
无肠上皮化生、异型以及恶性特征。
3.胃,胃底,活组织检查( STOMACH,FUNDUS, BIOPSY)
胃体型黏膜伴肠色素细胞样(enterochromaffin cell-like,ECL)细胞增生,通过嗜铬蛋白A(chromogranin A,CgA)和突触素(synaptophysin,Syn)免疫组化染色得以证实。见诊断评论。
萎缩性胃炎伴壁细胞完全性缺失、广泛肠上皮化生和幽门腺化生,形态学上提示为自身免疫性化生性萎缩性胃炎(autoimmune metaplastic atrophic gastritis,AMAG)。见诊断评论及参考文献。
幽门螺杆菌(Helicobacter pylori,HP)Giemsa染色呈阴性。
腺体无异型。
诊断评论:
第1部分和第3部分:胃体活组织检查显示萎缩性胃炎伴壁细胞缺失。存在幽门腺化生和广泛的肠上皮化生。无幽门螺杆菌感染。第2部分:胃窦活组织检查显示慢性非活动性胃炎伴G细胞增生,无肠上皮化生以及幽门螺杆菌感染。对胃体和胃窦活组织检查的发现高度提示自身免疫性化生性萎缩性胃炎(autoimmune metaplastic atrophic gastritis,AMAG)。AMAG是一种自身性抗体攻击壁细胞和/或主细胞性疾病,常见于年长患者。AMAG对胃窦通常无影响。临床上,AMAG患者可以表现为高胃泌素血症和/或恶性贫血。临床上建议检查CBC、血清胃泌素水平、抗壁细胞抗体、抗内因子抗体或维生素B12水平。
嗜铬蛋白A(chromogranin A,CgA)和突触素(synaptophysin,Syn)免疫组化染色证实,第1部分中存在类癌肿瘤。Ki-67免疫组化染色显示增殖指数约为1%。同时注意到在第1部分和第3部分背景中都存在ECL细胞增生。线性、小结节性、融合性微腺体中ECL细胞增生以及类癌的发生是胃泌素刺激ECL细胞致使组胺生成增加的结果。众所周知,发生于胃黏膜的化生性改变也是胃异型增生、腺瘤以及腺癌的高危因素。因此,AMAG患者应该进行内窥镜以及上面所提到的临床检查随访。
第2、3部分,Ki-67免疫组化染色突显了增生性腺体细胞。
参考文献:
1. Pitttman ME, Voltaggio L, Bhaijee F, Robertson SA, Montgomery EA. Autoimmune Metaplastic Atrophic Gastritis: Recognizing Precursor Lesions for Appropriate Patient Evaluation. Am J Surg Pathol. 2015 Aug 18. PMID: 26291507
2. Park JY, Cornish TC, Lam-Himlin D, Shi C, Montgomery E. Gastric lesions in patients with autoimmune metaplastic atrophic gastritis (AMAG) in a tertiary care setting. Am J Surg Pathol. 2010 Nov;34(11):1591-8. PMID: 20975338
3. Vanoli A, La Rosa S, Luinetti O, Klersy C, Manca R, Alvisi C, Rossi S, Trespi E, Zangrandi A, Sessa F, Capella C, Solcia E. Histologic changes in type A chronic atrophic gastritis indicating increased risk of neuroendocrine tumor development: the predictive role of dysplastic and severely hyperplastic enterochromaffin-like cell lesions. Hum Pathol. 2013 Sep;44(9):1827-37. PMID: 23642738
report for this case:
1. STOMACH, BODY, BIOPSY:
Gastric body-type mucosa with well-differentiated neuroendocrine tumor/carcinoid arising in a background of enterochromaffin cell-like (ECL) cell hyperplasia, confirmed by chromogranin A and synaptophysin immunostains. The proliferation index by Ki-67 is approximately 1%. See Diagnosis Comment.
Atrophic gastritis with complete loss of parietal cells, extensive intestinal metaplasia and pyloric metaplasia, morphologically suggestive of autoimmune metaplastic atrophic gastritis (AMAG). See Diagnosis Comment and references.
Negative for Helicobacter pylori on Giemsa stain.
Negative for glandular dysplasia.
2. STOMACH, ANTRUM, BIOPSY:
Gastric antral-type mucosa with chronic inactive gastritis.
Negative for Helicobacter pylori on both Giemsa stain and immunostain.
G-cell hyperplasia confirmed by synaptophysin and chromogranin A immunostains.
Negative for intestinal metaplasia, dysplasia, and malignancy.
3. STOMACH, FUNDUS, BIOPSY:
Gastric body-type mucosa with enterochromaffin cell-like (ECL) cell hyperplasia, confirmed by chromogranin A and synaptophysin immunostains. See Diagnosis Comment.
Atrophic gastritis with complete loss of parietal cells, extensive intestinal metaplasia and pyloric metaplasia, morphologically suggestive of autoimmune metaplastic atrophic gastritis (AMAG). See Diagnosis Comment and references.
Negative for Helicobacter pylori on Giemsa stain.
Negative for glandular dysplasia.
Diagnosis Comment:
For parts 1 and 3, the biopsies from gastric body show atrophic gastritis without parietal cells identified. Pyloric metaplasia and extensive intestinal metaplasia are present. No Helicobacter pylori identified. For part 2, a biopsy from gastric antrum shows chronic inactive gastritis with G-cell hyperplasia without intestinal metaplasia and without Helicobacter pylori infection. The findings from both gastric body and antrum are highly suggestive of autoimmune metaplastic atrophic gastritis (AMAG). AMAG is a disease which the antibodies attack the parietal cells and/or chief cells, and is usually seen in older patients. AMAG usually spares the attack of the gastric antrum. Clinically, the patient with AMAG may present as hypergastrinemia and/or pernicious anemia. Clinical work up is recommended to check CBC, serum gastrin level, anti-parietal cell antibody, anti-intrinsic factor antibody, or Vitamin B12 level.
Carcinoid tumor is identified in part 1 which is highlighted by synaptophysin and chromogranin A immunostains. The proliferative index is approximately 1% by Ki-67 immunostain. The ECL cell hyperplasia is noted in the background in both parts 1 and 3. Linear, micronodular, fused microglandular ECL cell hyperplasia and carcinoid development are the results of the stimulation of gastrin acting on ECL cells to increase histamine production, which eventually leads to nodular and linear ECL cell hyperplasia in patients with AMAG. Also, the metaplastic changes in the gastric mucosa are a known risk factor for gastric dysplasia, adenomas, and adenocarcinoma. Therefore, the patients with AMAG should be followed up endoscopically and clinically as mentioned above.
For parts 2 and 3, the Ki-67 immunostain highlights the proliferative glandular cells.
References:
1. Pitttman ME, Voltaggio L, Bhaijee F, Robertson SA, Montgomery EA. Autoimmune Metaplastic Atrophic Gastritis: Recognizing Precursor Lesions for Appropriate Patient Evaluation. Am J Surg Pathol. 2015 Aug 18. PMID: 26291507
2. Park JY, Cornish TC, Lam-Himlin D, Shi C, Montgomery E. Gastric lesions in patients with autoimmune metaplastic atrophic gastritis (AMAG) in a tertiary care setting. Am J Surg Pathol. 2010 Nov;34(11):1591-8. PMID: 20975338
3. Vanoli A, La Rosa S, Luinetti O, Klersy C, Manca R, Alvisi C, Rossi S, Trespi E, Zangrandi A, Sessa F, Capella C, Solcia E. Histologic changes in type A chronic atrophic gastritis indicating increased risk of neuroendocrine tumor development: the predictive role of dysplastic and severely hyperplastic enterochromaffin-like cell lesions. Hum Pathol. 2013 Sep;44(9):1827-37. PMID: 23642738

让每一家医院都拥有精细化亚专科服务;让人人享有便捷、准确、可靠的病理诊断服务。 疑难会诊咨询热线400-0098-600 柳芳芸 15968153692。
-
本帖最后由 芳芸 于 2015-11-18 15:28:37 编辑
孔老师语音点评:
最后诊断:1、自身免疫性萎缩性胃炎,2、类癌
自身免疫性化生性萎缩性胃炎(AMAG):是一种病理学表现为胃体、底部弥漫性萎缩,而胃窦黏膜基本正常的萎缩性胃炎,常伴有胃窦部G细胞增生,是胃神经内分泌肿瘤的常见前驱病变。
AMAG最显著的变化是胃泌酸性腺体萎缩甚至消失,其原因在于患者自身体内抗壁细胞和抗内因子抗体攻击并破坏壁细胞,导致盐酸的分泌量减少甚至缺如,这类患者几乎不会产生消化性溃疡的症状。相应的病理变化发生于存在壁细胞的区域,也就是胃底、体部之固有泌酸性腺体所在的部位。正如本例所示凡存在壁细胞的黏膜极度萎缩,代之以假幽门腺。有学者认为AMAG是发生在常染色体的显性遗传病,和幽门螺杆菌感染没有直接关系,但有研究者指出幽门螺杆菌感染可能是AMAG的早期诱发因素。病变常见于老年女性,临床常表现为恶性贫血,AMAG患者罹患胃癌的几率是其他人的3倍。同时还明确显示底体部正常泌酸性腺体萎缩、消失后取而代之的是化生性幽门腺腺体。胃窦部G细胞增生:正常情况下G细胞在胃内只分布于胃窦部腺体内,它是一种较大的核圆形的神经内分泌细胞,能够分泌胃泌素,但数量不多,平均每个腺体内含有4~5个G细胞。G细胞的增生可以是被动的也可以是主动的。本例是由于胃壁细胞减少,反馈性导致G细胞增生,是被诱导产生的。AMAG时增生的神经内分泌细胞主要是ECL细胞。目前已知胃内存在多种神经内分泌细胞成分,主要包括EC细胞、ECL细胞、G细胞、D细胞。EC细胞。
文字资料来源:中华病理学杂志2014年1月第43卷第1期

让每一家医院都拥有精细化亚专科服务;让人人享有便捷、准确、可靠的病理诊断服务。 疑难会诊咨询热线400-0098-600 柳芳芸 15968153692。