| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- Case 828 -- An 83 year old female with a lower GI bleed and multiple lesions
CLINICAL HISTORY
This 83 year old female with a history of non-small cell lung cancer status post left upper lobectomy and adjuvant chemotherapy, deep vein thrombosis and pulmonary embolism on coumadin, chronic obstructive pulmonary disease, and extensive sigmoid diverticulosis presented to an outside hospital with a lower GI bleed and found to have an ulcerated cecal mass, as well as, a polyp concerning for a villous adenoma during colonoscopy. She was stabilized, coumadin was held, and she was scheduled for outpatient follow up. Two weeks later, the patient presented again to the outside hospital emergency department with bright red blood per rectum and was subsequently transferred to UPMC Presbyterian for further management. The patient underwent a right hemicolectomy due to extensive blood loss and multiple peritoneal implants were noted during surgery
INTRAOPERATIVE DIAGNOSIS
PERITONEAL IMPLANT, BIOPSY (frozen section)
POSITIVE FOR MALIGNANCY CONSISTENT WITH POORLY DIFFERENTIATED CARCINOMA
-
本帖最后由 cqzhao 于 2014-07-04 01:05:06 编辑
- MIXED ADENONEUROENDOCRINE CARCINOMA OF THE CECUM, 5.0 CM, CONSISTING OF:
- MUCINOUS ADENOCARCINOMA WITH SIGNET RING CELL FEATURES.
- LARGE CELL NEUROENDOCRINE CARCINOMA (KI67 INDEX = 90%).
- TUMOR INVADES THROUGH THE VISCERAL PERITONEUM.
- POSITIVE FOR LYMPHOVASCULAR INVASION WITH LARGE VESSEL INVASION.
- PERINEURAL INVASION IS NOT IDENTIFIED.
- SURGICAL RESECTION MARGINS ARE NEGATIVE FOR MALIGNANCY AND DYSPLASIA.
- ONE OF ELEEVEN LYMPH NODES POSITIVE FOR METASTATIC CARCINOMA (1/11).
- PATHOLOGIC STAGE: pT4a N1a M1a.
- TWO TUBULAR ADENOMAS.
- Capella C., La Rosa S., Uccella S., Billo P., Cornaggia M. Mixed endocrine-exocrine tumors of the gastrointestinal tract. Semin. Diagn. Pathol. 2000;17:91-103.
- La Rosa S, Marando A, Sessa F, Capella C. Mixed adenoneuroendocrine carcinomas (MANECs) of the gastrointestinal tract: an update. Cancers. 2012;4:11-30.
- Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am. J. Surg. Pathol. 1987;11:S71-S86. doi: 10.1097/00000478-198700111-00007. [PubMed] [Cross Ref]
- Rindi G., Arnold R., Bosman F.T., Capella C., Kilmstra D.S., Kloppel G., Komminoth P., Solcia E. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman F.T., Carneiro F., Hruban R.H., Theise N.D., editors. WHO Classification of Tumours of the Digestive System. 4th. IARC Press; Lyon, France: 2010. pp. 13-14.
- Solcia E., Klöppel G., Sobin L.H. Histological Typing of Endocrine Tumours (WHO International Histological Classification of Tumours) 2nd. Springer; Berlin, Germany: 2000.
FINAL DIAGNOSIS
COLON, RIGHT, HEMICOLECTOMY:
DISCUSSION
R. Cardier first described gastrointestinal neuroendocrine tumors that contain an additional glandular component in 1924. Meanwhile, a number of similar cases have been reported using terms such as goblet cell carcinoid, composite carcinoid, adenocarcinoid, and small cell undifferentiated carcinoma which has led to confusion among clinicians and pathologists. These tumors are morphologically recognizable as having both glandular and neuroendocrine differentiation.
In the 2000 WHO classification of endocrine tumors, such neoplasms were defined as mixed exocrine-endocrine tumors when each component represents at least 30% of the lesion [5]. It is worth noting that adenocarcinomas with scattered neuroendocrine cells are not categorized as mixed exocrine-endocrine tumors, neither are neuroendocrine neoplasms with a focal glandular component since in most cases less than 30% of each component is present. Mixed exocrine-endocrine tumors are rare and are believed to account for less than 3% of colorectal neoplasms.
In 2010, the WHO classification for this neoplasm was again redefined as mixed adenoneuroendocrine carcinomas (MANECs). They may also be further classified as collision and composite types; where collision tumors consist of two different histologic patterns in close contact, and composite tumors composed of endocrine and exocrine cells that are intermixed within the same tumor.
The clinical significance of focal neuroendocrine differentiation in gastrointestinal adenocarcinomas is still somewhat debatable. Gastrointestinal mixed adenoneuroendocrine carcinomas (MANECs ) are a heterogeneous group of tumors showing different morphological, clinical, and prognostic features and can be grouped in different categories according to the grade of malignancy of each component [1]. When considering treatment, the more aggressive component of MANECs should be taken into consideration. Adenoneuroendocrine carcinomas containing a well differentiated neuroendocrine component and a glandular component should be treated as adenocarcinomas, whereas if a poorly differentiated neuroendocrine component is present, it should be treated as a gastrointestinal neuroendocrine carcinoma.
REFERENCES
![]() Contributed by Richard Freij, MD and Eizaburo Sasatomi, MD, PhD
Contributed by Richard Freij, MD and Eizaburo Sasatomi, MD, PhD
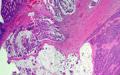
Immunohistochemical stains demonstrate that the solid nests of poorly differentiated tumor cells are diffusely positive for synaptophysin with weak to moderate staining intensity. CD56 is occasionally positive in scattered cells. Chromogranin is negative. The tumor cells of the mucinous adenocarcinoma component are largely negative for synaptophysin. Ki-67 proliferation marker is positive in approximately 90% of the tumor cells of both components.
Received is a right hemicolectomy (consisting of terminal ileum, 7.5 cm, cecum 3 cm, ascending colon, 18.5 cm, unremarkable omentum, 7.5 x 3 x 1.2 cm and mesentery ranging from 3.5 to 5 cm) specimen measuring approximately 25 cm in overall length with open circumferences of 4 cm (proximal) and 7 cm (distal).
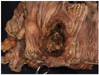
A deep ulcerating, slightly raised rolled rimmed, oval, necrotic, 5 x 3 cm, tumor is located within 1 cm of the ileocecal valve and 14 cm from the distal resection margin. On cross-section, the ivory firm proximal ascending colon tumor is near circumferential, 95%, and up to 1.8 cm thick and extends to the serosal wall. The tumor underlies the ileocecal valve and small bowel mucosa (involves submucosa), and is 3 cm from the mesenteric resection margin. The serosal surface is hemorrhage with scant tan exudate, but, without obvious perforation. Multiple potential tumor involved lymph nodes are noted with possible tumor invading vasculature. The largest potential lymph node measures 0.6 cm.
There are five, tan, sessile polyps ranging in size from 0.4-0.5 cm with a single pedunculated polyp measuring 0.6 x 0.5 x 0.3 cm the polyps are located within 1 to 6 cm distal of the ascending colon mass. The remaining mucosa appears unremarkable.