| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- B62右侧卵巢囊肿-透明细胞癌
| 性别 | 女 | 年龄 | 68岁 | 临床诊断 | |
|---|---|---|---|---|---|
| 一般病史 | B超发现右侧卵巢囊肿 | ||||
| 标本名称 | 右侧卵巢囊肿 | ||||
| 大体所见 | 囊壁样组织一块,4X4X2CM,局部见1.5X1.5cm大小乳头状物。 | ||||

名称:图1
描述:A239

名称:图2
描述:A240

名称:图3
描述:A241

名称:图4
描述:A242

名称:图5
描述:A243

名称:图6
描述:A244

名称:图7
描述:A245

名称:图8
描述:A246

名称:图9
描述:A247

名称:图10
描述:A248

名称:图11
描述:A249

名称:图12
描述:A250

名称:图13
描述:A251

名称:图14
描述:A252

名称:图15
描述:A253

名称:图16
描述:A254

名称:图17
描述:A255

名称:图18
描述:A256

名称:图19
描述:A257

名称:图20
描述:A258

名称:图21
描述:A259

名称:图22
描述:A260

名称:图23
描述:A261

名称:图24
描述:A262

名称:图25
描述:A263

名称:图26
描述:A264

名称:图27
描述:A265

名称:图28
描述:A266

名称:图29
描述:A267

名称:图30
描述:A268

名称:图31
描述:A269

名称:图32
描述:A270

名称:图33
描述:A271

名称:图34
描述:A272

名称:图35
描述:A273

名称:图36
描述:A274

名称:图37
描述:A275

名称:图38
描述:A276

名称:图39
描述:A277

名称:图40
描述:A278

名称:图41
描述:A279

名称:图42
描述:A280

名称:图43
描述:A281

名称:图44
描述:A282
-
本帖最后由 Renghis 于 2013-04-27 17:42:42 编辑

- 重归学生时代!
分型:囊性型ccc;腺纤维瘤型ccc
另外一种:伴有腺纤维瘤型CCC;不伴有腺纤维瘤型ccc
下面的文献关于子宫内膜异位病与CCC的关系以及分型、预后等等内容;(似乎有矛盾的结论)
Cystic and adenofibromatous clear cell carcinomas of the ovary: distinctive tumors that differ in their pathogenesis and behavior: a clinicopathologic analysis of 122 cases.
Source
Department of Pathology, The Johns Hopkins University School of Medicine and Hospital, Baltimore, MD, USA. verase@mskcc.org
Abstract
Ovarian clear cell carcinomas (CCC) typically present as large adnexal, stage I tumors and are generally considered highly malignant. They are frequently associated with endometriosis and, less often with clear cell adenofibromas. We hypothesized that CCCs are a heterogeneous group of tumors, some arising from a cyst and others from an adenofibroma. To test this hypothesis, 122 cases of CCC were retrieved from the surgical pathology files of National Taiwan University Hospital (74), The Johns Hopkins Hospital (23), and Serei Mikatahara General Hospital (23) (1985 to 2006). Cases were divided into 3 subgroups: (1) cystic, (2) adenofibromatous, and (3) indeterminate. Various features were analyzed including: age, race, laterality, tumor size, architectural pattern (papillary, tubulo-cystic, solid, mixed patterns), grade, mitotic index, association with endometriosis including atypical endometriosis/intraepithelial carcinoma, stage and survival. Nearly 70% of all the patients were diagnosed as stage I. The 2-year and 5-year survival (all stages) was 78% and 68%, respectively. Striking clinicopathologic differences were observed between cystic and adenofibromatous CCCs. Cystic CCC was more frequently diagnosed as stage I compared with adenofibromatous CCC (75% vs. 44%). Conversely, adenofibromatous CCCs were diagnosed more often in advanced stages (stages II-IV) compared with cystic CCCs (56% vs. 18%). Both the cystic and adenofibromatous CCC forms were associated with endometriosis and atypical endometriosis/intraepithelial carcinoma, but the frequency was much higher in the cystic group. Specifically, endometriosis was found in 91% of cystic CCCs and atypical endometriosis/intraepithelial carcinoma in 62% of these cases, whereas endometriosis was found in 44% of adenofibromatous CCCs and atypical endometriosis/intraepithelial carcinoma in 11% of cases. A predominantly papillary pattern was seen in 47% of cystic CCCs, whereas none of the adenofibromatous carcinomas displayed a predominantly papillary pattern. A more favorable outcome was observed for cystic CCCs compared with adenofibromatous CCCs (all stages) which was accounted for by the high proportion of stage I tumors. The 2-year and 5-year survival for the cystic CCCs was 82% and 77% and for the adenofibromatous CCCs (all stages), 62% and 37%, respectively. In summary, subdividing ovarian CCCs into cystic and adenofibromatous CCC reveals differences in a number of clinicopathologic features including their association with endometriosis, histologic patterns, stage distribution, and clinical behavior. Because there were a relatively small number of adenofibromatous CCCs in this series, additional cases must be studied to confirm these findings.
Clear cell adenocarcinoma associated with clear cell adenofibromatous components: a subgroup of ovarian clear cell adenocarcinoma with distinct clinicopathologic characteristics.
Source
Department of Basic Pathology, National Defense Medical College, 3-2 Namiki, Tokorozawa, Saitama 359-8513, Japan.
Abstract
We occasionally encountered clear cell adenofibromatous (CCAF) components coexisting in the ovarian clear cell adenocarcinoma (CCA). To reveal the clinicopathologic significance of CCAF components in CCA, we classified 67 cases of surgically resected CCA into CCA with and without CCAF components [CCAF(+) and (-) groups], and compared clinicopathologic parameters, that is, patient age, clinical stage, the degree of optimal cytoreduction, patient outcome, histologic grade and Ki-67 labeling index of the CCA, and the presence of endometriosis, between these 2 groups. Fourteen cases (21%) and 53 cases were classified as CCAF(+) and CCAF(-) groups, respectively. Of these 14 CCAF(+) cases, the CCAF components with atypia were observed adjacent to the CCAF components without atypia in 10, and adjacent to the obvious CCAs in 13 cases. In comparison with the CCAF(-) group, the CCAF(+) group showed a higher frequency of histologically low-grade tumors [93% (13 of 14) vs. 43% (23 of 53), P=0.0027], a lower Ki-67 labeling index (mean 35.9% vs. 44.0%, P=0.0492), and better patient prognosis (5-year survival 78.8% vs. 49.3%, P=0.0277). Endometriosis was much less frequent in the CCAF(+) group than in the CCAF(-) group [14.7% (2 of 14) vs. 67.9% (36 of 53), P=0.00096]. Multivariate analysis identified only optimal cytoreduction as independent favorable prognostic factor. These results suggest that CCAF besides endometriosis is associated with the development of CCA, and that the CCAF(+) group may be a distinct subgroup of CCA with less aggressive biologic behavior.
Cancer. 2011 Feb 21;2:94-106.
Pathogenesis of ovarian clear cell adenofibroma, atypical proliferative (borderline) tumor, and carcinoma: clinicopathologic features of tumors with endometriosis or adenofibromatous components support two related pathways of tumor development.
Source
1. Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, PA, USA;
Abstract
The clinicopathologic features of 472 ovarian epithelial clear cell neoplasms (4 adenofibromas [AFs], 41 atypical proliferative [borderline] tumors [APTs], and 427 carcinomas [CAs]) were studied in order to elucidate the morphologic steps involved in the pathogenesis of these tumors and determine whether clear cell CA is a type I or type II tumor in the dualistic model of ovarian carcinogenesis. Thirty-three percent of the CAs had an adenofibromatous background [CA(AF+)], and 67% did not [CA(AF-)]. Endometriosis was found in all types of tumors, but tumors arising in endometriotic cysts were more frequent with CA(AF-)s (p<0.0001). The subset of women with CA(AF-)s with endometriosis were younger (p<0.0001), their tumors were more frequently cystic (p<0.0001), they more commonly had a mixed carcinoma component of non-clear cell type (p=0.006), and they were more frequently oxyphilic (p=0.015) compared with CA(AF+)s. The architecture of the former tumors was more commonly papillary compared to tubulocystic in the latter (p=0.0006). Atypical endometriosis was more common in CA(AF-)s than in AFs, APTs, and CC(AF+)s [p=0.004]. The subset of CA(AF-)s without endometriosis presented more frequently in advanced stage (>I) and were higher grade compared to CA(AF+)s or CA(AF-) with endometriosis (p-values, <0.0001 to 0.0071). All AFs and APTs were stage I compared to 79% of CA(AF+)s. An increase in mean tumor size correlated with each respective tumor category from AF (6.8 cm) to CA(AF+) [12.9 cm]. Notable nuclear atypia was absent in all AFs but was focally present in 27% of APTs and in the adenofibromatous background of 24% of the CA(AF+)s. An increase in the proportion of carcinoma in the CA(AF+)s correlated with an increase in grade and advanced stage. In summary, ovarian clear cell CA appears to develop along two pathways, both of which are related to endometriosis. We speculate that, in one, epithelial atypia arises in an endometriotic cyst and then evolves into clear cell CA, and, in the other, non-cystic endometriosis induces a fibromatous reaction resulting in the formation of AF, which then develops into APT and subsequently a clear cell CA. The absence of endometriosis or adenofibromatous components in CC(AF-)s may be due to overgrowth and obliteration by the invasive carcinoma. Finally, the findings in this study support the view that both types of clear cell CA [CC(AF+) and CC(AF-)] are more closely related to type I tumors.
Thank TK1905 to fine these three related papers. The last one is my paper which is the largest study in this area. I spent several years to review all the slides from AFIP. Unfortunately i cannot find the follow-up results for these old cases and cannot make the paper more significant.
This is an excellent teaching case. In term of pathogenesis of clear cell ca, most people think it can arise from endometriosis. In fact this is another pathway benign clear cell adenofibroma-----clear cell borderline tumor-----clear cell carcinoma. You can find all the three components for this case.
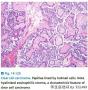
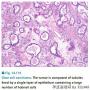
Clear cell carcinomas display several different patterns, include papillary, tubulocystic, and solid. The papillary pattern is characterized by papillae that are either fibrotic but more often are hyalinized. In fact, the hyalinized papillary cores are a very characteristic feature of this tumor. The tubulocystic pattern is characterized by varying size tubules and cysts. The majority of tumors display combinations of all of these patterns.
受各位老师开导,特别是TK1905 老师的精彩文献引用,本人认同卵巢透明细胞癌。

- 重归学生时代!
分型:囊性型ccc;腺纤维瘤型ccc
另外一种:伴有腺纤维瘤型CCC;不伴有腺纤维瘤型ccc
下面的文献关于子宫内膜异位病与CCC的关系以及分型、预后等等内容;(似乎有矛盾的结论)
Cystic and adenofibromatous clear cell carcinomas of the ovary: distinctive tumors that differ in their pathogenesis and behavior: a clinicopathologic analysis of 122 cases.
Source
Department of Pathology, The Johns Hopkins University School of Medicine and Hospital, Baltimore, MD, USA. verase@mskcc.org
Abstract
Ovarian clear cell carcinomas (CCC) typically present as large adnexal, stage I tumors and are generally considered highly malignant. They are frequently associated with endometriosis and, less often with clear cell adenofibromas. We hypothesized that CCCs are a heterogeneous group of tumors, some arising from a cyst and others from an adenofibroma. To test this hypothesis, 122 cases of CCC were retrieved from the surgical pathology files of National Taiwan University Hospital (74), The Johns Hopkins Hospital (23), and Serei Mikatahara General Hospital (23) (1985 to 2006). Cases were divided into 3 subgroups: (1) cystic, (2) adenofibromatous, and (3) indeterminate. Various features were analyzed including: age, race, laterality, tumor size, architectural pattern (papillary, tubulo-cystic, solid, mixed patterns), grade, mitotic index, association with endometriosis including atypical endometriosis/intraepithelial carcinoma, stage and survival. Nearly 70% of all the patients were diagnosed as stage I. The 2-year and 5-year survival (all stages) was 78% and 68%, respectively. Striking clinicopathologic differences were observed between cystic and adenofibromatous CCCs. Cystic CCC was more frequently diagnosed as stage I compared with adenofibromatous CCC (75% vs. 44%). Conversely, adenofibromatous CCCs were diagnosed more often in advanced stages (stages II-IV) compared with cystic CCCs (56% vs. 18%). Both the cystic and adenofibromatous CCC forms were associated with endometriosis and atypical endometriosis/intraepithelial carcinoma, but the frequency was much higher in the cystic group. Specifically, endometriosis was found in 91% of cystic CCCs and atypical endometriosis/intraepithelial carcinoma in 62% of these cases, whereas endometriosis was found in 44% of adenofibromatous CCCs and atypical endometriosis/intraepithelial carcinoma in 11% of cases. A predominantly papillary pattern was seen in 47% of cystic CCCs, whereas none of the adenofibromatous carcinomas displayed a predominantly papillary pattern. A more favorable outcome was observed for cystic CCCs compared with adenofibromatous CCCs (all stages) which was accounted for by the high proportion of stage I tumors. The 2-year and 5-year survival for the cystic CCCs was 82% and 77% and for the adenofibromatous CCCs (all stages), 62% and 37%, respectively. In summary, subdividing ovarian CCCs into cystic and adenofibromatous CCC reveals differences in a number of clinicopathologic features including their association with endometriosis, histologic patterns, stage distribution, and clinical behavior. Because there were a relatively small number of adenofibromatous CCCs in this series, additional cases must be studied to confirm these findings.
Clear cell adenocarcinoma associated with clear cell adenofibromatous components: a subgroup of ovarian clear cell adenocarcinoma with distinct clinicopathologic characteristics.
Source
Department of Basic Pathology, National Defense Medical College, 3-2 Namiki, Tokorozawa, Saitama 359-8513, Japan.
Abstract
We occasionally encountered clear cell adenofibromatous (CCAF) components coexisting in the ovarian clear cell adenocarcinoma (CCA). To reveal the clinicopathologic significance of CCAF components in CCA, we classified 67 cases of surgically resected CCA into CCA with and without CCAF components [CCAF(+) and (-) groups], and compared clinicopathologic parameters, that is, patient age, clinical stage, the degree of optimal cytoreduction, patient outcome, histologic grade and Ki-67 labeling index of the CCA, and the presence of endometriosis, between these 2 groups. Fourteen cases (21%) and 53 cases were classified as CCAF(+) and CCAF(-) groups, respectively. Of these 14 CCAF(+) cases, the CCAF components with atypia were observed adjacent to the CCAF components without atypia in 10, and adjacent to the obvious CCAs in 13 cases. In comparison with the CCAF(-) group, the CCAF(+) group showed a higher frequency of histologically low-grade tumors [93% (13 of 14) vs. 43% (23 of 53), P=0.0027], a lower Ki-67 labeling index (mean 35.9% vs. 44.0%, P=0.0492), and better patient prognosis (5-year survival 78.8% vs. 49.3%, P=0.0277). Endometriosis was much less frequent in the CCAF(+) group than in the CCAF(-) group [14.7% (2 of 14) vs. 67.9% (36 of 53), P=0.00096]. Multivariate analysis identified only optimal cytoreduction as independent favorable prognostic factor. These results suggest that CCAF besides endometriosis is associated with the development of CCA, and that the CCAF(+) group may be a distinct subgroup of CCA with less aggressive biologic behavior.
Cancer. 2011 Feb 21;2:94-106.
Pathogenesis of ovarian clear cell adenofibroma, atypical proliferative (borderline) tumor, and carcinoma: clinicopathologic features of tumors with endometriosis or adenofibromatous components support two related pathways of tumor development.
Source
1. Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, PA, USA;
Abstract
The clinicopathologic features of 472 ovarian epithelial clear cell neoplasms (4 adenofibromas [AFs], 41 atypical proliferative [borderline] tumors [APTs], and 427 carcinomas [CAs]) were studied in order to elucidate the morphologic steps involved in the pathogenesis of these tumors and determine whether clear cell CA is a type I or type II tumor in the dualistic model of ovarian carcinogenesis. Thirty-three percent of the CAs had an adenofibromatous background [CA(AF+)], and 67% did not [CA(AF-)]. Endometriosis was found in all types of tumors, but tumors arising in endometriotic cysts were more frequent with CA(AF-)s (p<0.0001). The subset of women with CA(AF-)s with endometriosis were younger (p<0.0001), their tumors were more frequently cystic (p<0.0001), they more commonly had a mixed carcinoma component of non-clear cell type (p=0.006), and they were more frequently oxyphilic (p=0.015) compared with CA(AF+)s. The architecture of the former tumors was more commonly papillary compared to tubulocystic in the latter (p=0.0006). Atypical endometriosis was more common in CA(AF-)s than in AFs, APTs, and CC(AF+)s [p=0.004]. The subset of CA(AF-)s without endometriosis presented more frequently in advanced stage (>I) and were higher grade compared to CA(AF+)s or CA(AF-) with endometriosis (p-values, <0.0001 to 0.0071). All AFs and APTs were stage I compared to 79% of CA(AF+)s. An increase in mean tumor size correlated with each respective tumor category from AF (6.8 cm) to CA(AF+) [12.9 cm]. Notable nuclear atypia was absent in all AFs but was focally present in 27% of APTs and in the adenofibromatous background of 24% of the CA(AF+)s. An increase in the proportion of carcinoma in the CA(AF+)s correlated with an increase in grade and advanced stage. In summary, ovarian clear cell CA appears to develop along two pathways, both of which are related to endometriosis. We speculate that, in one, epithelial atypia arises in an endometriotic cyst and then evolves into clear cell CA, and, in the other, non-cystic endometriosis induces a fibromatous reaction resulting in the formation of AF, which then develops into APT and subsequently a clear cell CA. The absence of endometriosis or adenofibromatous components in CC(AF-)s may be due to overgrowth and obliteration by the invasive carcinoma. Finally, the findings in this study support the view that both types of clear cell CA [CC(AF+) and CC(AF-)] are more closely related to type I tumors.
这个应该是直接诊断透明细胞癌。
若考虑浆液性肿瘤只能算交界性浆液性肿瘤伴细胞透明变,这也是本例挑战之处,因为一旦这样诊断就意味着一个诊断是恶性,另外一个诊断是交界性,对病人影响大
本例明显有透明细胞腺纤维瘤样成分,所以那些乳头就是透明细胞癌的证据了,乳头用浆液上皮解释最多够交界,但是用透明细胞源性解释就直接是浸润型透明细胞癌啦。
透明和浆液都可以有乳头和鞋钉结构,胞浆都可以嗜酸性,确实蛮难鉴别的,尤其浆液还可以伴有细胞透明变的时候。
本例乳头区域有明显的玻璃样变性的乳头纤维轴心,这点或许是透明细胞癌与浆液性肿瘤鉴别的一个重要参考点
交界性的透明细胞腺纤维瘤、交界透明细胞腺纤维瘤伴有微浸润以及良性的透明细胞腺纤维瘤都是极其罕见的病例,文献或报道的例子极少或并未有很好建立其标准。
还有:刚开始看还以为是交界性透明细胞腺纤维瘤合并交界性浆液性囊乳头状腺瘤呢!其实一个透明细胞癌可以解释全部形态
最后:靠IHC了
学习了,谢谢

谢
-
本帖最后由 TK1905 于 2013-04-09 21:46:51 编辑
这个应该是直接诊断透明细胞癌。
若考虑浆液性肿瘤只能算交界性浆液性肿瘤伴细胞透明变,这也是本例挑战之处,因为一旦这样诊断就意味着一个诊断是恶性,另外一个诊断是交界性,对病人影响大
本例明显有透明细胞腺纤维瘤样成分,所以那些乳头就是透明细胞癌的证据了,乳头用浆液上皮解释最多够交界,但是用透明细胞源性解释就直接是浸润型透明细胞癌啦。
透明和浆液都可以有乳头和鞋钉结构,胞浆都可以嗜酸性,确实蛮难鉴别的,尤其浆液还可以伴有细胞透明变的时候。
本例乳头区域有明显的玻璃样变性的乳头纤维轴心,这点或许是透明细胞癌与浆液性肿瘤鉴别的一个重要参考点
交界性的透明细胞腺纤维瘤、交界透明细胞腺纤维瘤伴有微浸润以及良性的透明细胞腺纤维瘤都是极其罕见的病例,文献或报道的例子极少或并未有很好建立其标准。
还有:刚开始看还以为是交界性透明细胞腺纤维瘤合并交界性浆液性囊乳头状腺瘤呢!其实一个透明细胞癌可以解释全部形态
最后:靠IHC了
贴个交界透明细胞肿瘤的内容:
Occasionally, an endometriotic cyst is lined by atypical are virtually no published data on clear cell tumors with 透明细胞癌 Clear cell carcinomas display several different patterns,
APCCT comprise 0.2% of ovarian epithelial tumors
(> ). Among approximately 30 cases of
APCCT (clear cell adenofibroma of borderlinemalignancy
or low malignant potential) in the literature, the mean age
is 60–70 years. The mean tumor diameter is about 15 cm.
Microscopically, the architecture is similar to the
clear cell adenofibroma, but glands aremore crowded. The
tumor has greater epithelial proliferation and atypia than
the adenofibroma and lacks stromal invasion. The cell
types lining the glands and cystic spaces are similar to
those in benign tumors but display significant nuclear
atypia with coarse chromatin clumping, prominent nucle-
oli, and mitotic activity up to 3 per 10 HPF. The epithe-
liummay display stratification and budding; true papillary
structures are uncommon. Small solid nests of clear cells,
significant gland crowding, or papillary growth should
raise the suspicion of stromal invasion. Distinction of an
APCCT from clear cell carcinoma is one of the most
difficult distinctions in gynecologic pathology. Some
clear cell carcinomas lack an obviously infiltrative pattern
and are characterized solely by crowded glands. The point
at which gland crowding in a clear cell neoplasm reflects
invasion is not well defined. The vast majority of tumors
in which this differential is considered are usually classi-
fied as clear cell carcinoma. This practice helps ensure
comprehensive staging where metastases might be found
and thereby confirm a diagnosis of carcinoma. Further-
more, it gives the patient the potential benefit of chemo-
therapy if there is doubt about the diagnosis.
epithelial cells with clear cytoplasm. This has been desig-
nated ‘‘atypical endometriosis’’ (see>Chap. 13, Diseases
of the Peritoneum)[298]. Rarely, cytologic features of
malignancy are present in this setting. If the lesion is well
sampled and invasion is not found, the lesion is classified
as APCCT with intraepithelial carcinoma. Microinvasion
should be diagnosed if invasive areas measure less than
5 mm, but this finding should prompt additional sam-
pling to look for diagnostic features of carcinoma. There
microinvasion or intraepithelial carcinoma, both of which
are exceedingly rare.
The rarity of clear cell adenofibroma and APCCT
could reflect the fact that precursors of clear cell carci-
noma most often have the morphology of endometriosis
with atypia rather than a clear cell neoplasm. Peritoneal
‘‘implants’’ have not been described with APCCTs. Among
the limited reported APCCTs, there is one alleged recur-
rence and no tumor deaths.
which often occur together (> – ).
These include papillary, tubulocystic, and solid. In
a minority of cases, there is a prominent
adenofibromatous component [369](> and
> ). Recently, some investigators have subdivided
clear cell carcinomas into those arising in a cyst and
those that have an adenofibromatous background [369].
The cystic tumors are more frequently papillary, whereas
the tubulocystic pattern tends to dominate in the
adenofibromatous tumors. The cystic variant is more fre-
quently associated with endometriosis. In addition, the
behavior of the two types of tumors may be different
although the data have been conflicting [369]. These find-
ings suggest that the two variants arise along different
pathways.
The solid pattern of clear cell carcinoma is character-
ized by sheets of polyhedral cells with abundant, clear
cytoplasm separated by delicate fibrovascular septae or dense fibrotic stroma. The papillary pattern is character-
ized by papillae that are either fibrotic but more often are
hyalinized (>Figs. 14.116, >14.117, and >14.120). In
fact, the hyalinized papillary cores are a very characteristic
feature of this tumor. The tubulocystic pattern is charac-
terized by varying size tubules and cysts (>Fig. 14.114).
The majority of tumors display combinations of all of
these patterns [369]. Despite being designated clear cell
carcinoma, many of the cells comprising clear cell carci-
noma contain slightly granular eosinophilic cytoplasm.