Infiltrating Syringomatous Adenoma of the Nipple: Clinical Presentation and Literature Review
From the Departments of Hematology/Oncology (Dr Oo) and Pathology (Dr Xiao), The Brooklyn Hospital Center, Brooklyn, New York.
| Abstract |
|---|
Infiltrating syringomatous adenoma of the nipple is a rare neoplasm of the breast. Syringomatous adenoma of the nipple is often misdiagnosed because clinical examination and mammographic findings of syringomatous adenoma of the nipple mimic carcinoma. Despite its benign behavior, syringomatous adenoma of the nipple usually shows infiltrative expansile proliferation into adjacent tissue and underlying breast tissue. Up until now, to our knowledge, there has been no reported case of regional or distant metastasis. Histologically and clinically, syringomatous adenoma of the nipple is often confused with tubular carcinoma as well as low-grade adenosquamous carcinoma of the breast. Special attention given to this tumor by pathologists and clinicians can avoid misdiagnosis and unnecessary treatment.
Accepted: November 7, 2008
Rosen1 described syringomatous adenoma of the nipple (SAN) in 1983. SAN is morphologically similar to syringoma, a tumor of eccrine duct origin. Although “derivation from eccrine structures of the nipple” has been proposed, the true origin of SAN remains unknown. It may originate from a pluripotential adnexal keratinocyte capable of both follicular and sweat gland differentiation.2 To the best of our knowledge, only 34 cases of SAN have been reported in the literature.1–11 Syringomatous adenoma of the nipple is locally infiltrating and does not metastasize. Appropriate local treatment depends on an accurate diagnosis. In this article, we discuss the clinical presentation, pathologic features, differential diagnosis, treatment options, and prognosis of this rare entity.
| CLINICAL PRESENTATION |
|---|
Syringomatous adenoma of the nipple typically presents as a solitary firm mass in the subareolar or nipple region of unilateral breast. Syringomatous adenoma of the nipple also occurs in breast parenchyma and in bilateral breasts.5,12 The size ranges from 1 cm to 3 cm in diameter. Patients may have pain, tenderness, itchiness, crusting, ulceration, and nipple discharge or nipple inversion.
The mean age at presentation is 40 years, and the age range is 11 to 76 years. All but one case of SAN occurred in women.1 Mammography of SAN generally demonstrates a mass in the subareolar region with dense and irregular outline, spicular formation and foci of microcalcifications. Ultrasonography shows an ill-defined mass with heterogeneous internal echoes. Radiographic findings suggest malignancy. Infiltrating SAN is indistinguishable from carcinoma on mammography or ultrasound imaging examination.12 Because it is often difficult to correctly diagnose SAN using fine-needle aspiration or needle biopsy, many cases are reported as suspicious for malignancy.
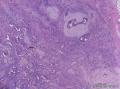
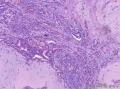
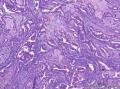
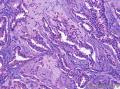
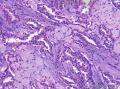
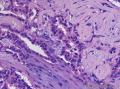
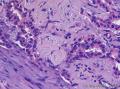
Grossly, SAN varies in size from 1 to 3 cm in diameter. Cut section shows an ill-defined tumor with minute cystic spaces around the nipple beneath the epidermis. The tumor is firm and homogeneously white. The areolar skin and underlying breast tissue are unremarkable. Microscopically, SAN has an infiltrating pattern characterized by nests and branching cords of cells forming glandular structures. The tumor cells infiltrate the stroma between smooth muscle bundles (Figure 1, A and B). They also invade perineural region.4,6 Small, keratinous cysts are present around the skin surface. These ducts are lined by 2 layers of cells and had a teardrop, tadpole, or comma shape, giving the general impression of a syringoma. At higher magnification, a distinct layer of myoepithelial cells can be identified between the epithelial cells and the fibrous stroma (Figure 1, C and D). The surrounding breast tissue might appear normal or show hyperplastic changes. Syringomatous adenoma of the nipple is not associated with the overlying skin or nipple epidermis involvement.
Histopathologic diagnostic criteria of SAN include (1) location in dermis and subcutis of nipple or areola; (2) irregular, compressed, or comma-shaped tubules infiltrating into smooth muscle bundles and/or nerves; (3) presence of myoepithelial cells around the tubules; (4) presence of cysts lined by stratified squamous epithelium and filled with keratinous material; and (5) absence of mitotic activity and necrosis.
It is difficult to diagnose SAN by a stereotactic core breast biopsy or a fine-needle aspiration.13 Syringomatous adenoma of the nipple can be misinterpreted as a carcinoma on frozen section or needle biopsy, resulting in unnecessary mastectomy and axillary dissection.3,7
Syringomatous adenoma of the nipple is histologically similar to a syringoma, a benign tumor originating in the ducts of the dermal sweat glands. Squamous metaplasia may be present in both SAN and nipple adenoma. Thus, SAN can often be mistaken for nipple adenoma, a benign variant of intraductal papilloma associated with serous or bloody nipple discharge. Microscopically, the nipple adenoma exhibits epithelial hyperplasia arising from a lactiferous duct displacing the nipple stroma. Syringomatous adenoma of the nipple, on the other hand, displays stromal infiltration. In nipple adenoma, myoepithelial cells are uniformly present.14 Because of its clinical presentation of nipple ulceration and crusting, in one particular case, SAN was misjudged as Paget disease, prompting unnecessary mastectomy.3
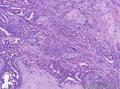
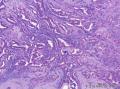
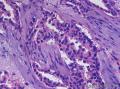
Syringomatous adenoma of the nipple has often been mistaken for tubular carcinoma of the breast. Angular, pointed glands of tubular carcinoma are similar to the teardrop or comma-shaped tubules of SAN. Although tubular carcinomas demonstrate more than 90% tubules with low-grade features and open lumina with apocrine-like snouts and basophilic secretions (Figure 1, E and F), SAN generally shows compressed lumina (Figure 1, B). Most tubular carcinomas are associated with micropapillary or cribriform types of low-grade ductal carcinoma in situ. The presence of squamous metaplasia helps differentiate SAN from tubular carcinoma. Basement membrane and myoepithelial cells are absent in tubular carcinoma (Figure 1, F). Immunohistochemical stain for p63 and/or smooth muscle myosin heavy chain can be useful in difficult cases (Figure 1, G).
Syringomatous adenoma of the nipple can also be confused with low-grade adenosquamous carcinoma of the breast, which is a well-differentiated tumor with both glandular and squamous differentiation. Low magnification reveals compressed lumens with infiltrating growth pattern in both conditions. Syringomatous adenoma of the nipple is benign and does not metastasize, whereas low-grade adenosquamous carcinoma of the breast is considered a low-grade variant of metaplastic carcinoma of breast with evidence of metastasis.15
The management of SAN is complete local excision to achieve histologically negative margins. Syringomatous adenoma of the nipple recurs if not totally excised. In patients having negative margins after the removal of a whole SAN, there was no evidence of recurrence during a follow-up period of 1 to 6 years.5 However, those patients having positive margins, after local surgical excision, experienced tumor recurrence.4 Therefore, careful monitoring to detect local recurrence is considered necessary.9,16 Most of the recurrences were managed with local reexcision. Recently, reconstruction after central mound resection has evolved as an alternative therapy.10 In spite of its local aggressiveness and recurrence, SAN is not known to metastasize.4,5
| CONCLUSION |
|---|
Syringomatous adenoma of the nipple is a benign, but locally aggressive, tumor that is often misdiagnosed as tubular carcinoma. The unusual clinical presentations and the mammographic findings of SAN can potentially present falsely as “highly suspicious of malignancy.” Therefore, this benign lesion deserves special attention by pathologists and clinicians to avoid misdiagnosis and unnecessary surgery.