| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- 女,33岁,左颞部肿块(1126819)
Carcinoma Ex Pleomorphic Adenoma早已有翻译——癌在多形性腺瘤中。
本例是良性的,无恶性特征。汗腺瘤、软组织混合瘤可以这样。有多种分化而已。有些囊性区域,有些透明细胞汗腺瘤区域
谢谢您的提示和翻译,EX这个可以作为词根也可以作为单词来看,是从。。。来的,不是中的意思啊,就像是溃疡恶变和癌溃疡的概念。这个概念更倾向“发生自(来自)多形性腺瘤的癌”或是“癌从多形性腺瘤发生的”或是“由多形性腺瘤发展来的癌”或是“癌由多形性腺瘤发展而来”,这样强调的是在多形性腺瘤发生和发展基础上由于其瘤内腺上皮和肌上皮发生恶性癌变从而发展形成的恶性肿瘤成分,这个因果关系和“癌在多形性腺瘤中”的概念是不同,后者有可能是二元论的感觉,不能够完全提示病变的因果关系,也不能很好的给临床提供多形性腺瘤癌变的潜在风险性。个人看法啊,主要是我当时看到咱们网上的两个病例和网上的文献,自己本身感觉这个概念不是很好理解,所以说说自己的看法,欢迎大家拍砖啊,呵呵
哈哈,就本例。我的感觉是就形态学论形态学的角度,多形性腺瘤中的粘液性上皮成分7XIW%25OLG.jpg) 周围可见基底细胞样类似肌上皮成分或中间细胞样或是表皮细胞样的区域,还是非常符合粘液表皮样癌的形态学的,这样成分的出现应该是出现在病例报告内,因为多形性腺瘤的病人必然会面对着复发甚至长期存在年龄大了以后的其他可能。因为肿瘤体积中等,估计楼主看到的包膜情况是乐观的,所以病人本身的预后是乐观的,但是随访也是应该和必须提醒病人的。
周围可见基底细胞样类似肌上皮成分或中间细胞样或是表皮细胞样的区域,还是非常符合粘液表皮样癌的形态学的,这样成分的出现应该是出现在病例报告内,因为多形性腺瘤的病人必然会面对着复发甚至长期存在年龄大了以后的其他可能。因为肿瘤体积中等,估计楼主看到的包膜情况是乐观的,所以病人本身的预后是乐观的,但是随访也是应该和必须提醒病人的。
-
本帖最后由 limo 于 2011-11-18 17:40:20 编辑
http://www.ipathology.cn/channel/detail/name/bbs/article/404022.html
两例互为补充,不知道年龄有没有可比性,本例年龄轻,可能还需要多取材做工作。文献说发生率在多形性腺瘤中占6%,一般年龄比较大,60岁以上,30岁以下比较少见,就是这个Carcinoma Ex Pleomorphic Adenoma 咋翻译呢啊,咱们翻译为恶性多形性腺瘤但是感觉还是有点信息不足的问题 “EX”是个啥意思呢。
所以本例谨慎,但是另外一例又怎样呢。
镜下主要是
The most common malignant epithelial subtype seen in carcinoma ex pleomorphic adenoma is poorly differentiated adenocarcinoma not otherwise specified (NOS)最常见的是低分化癌非特指. Almost all other malignant varieties of salivary gland tumors have been described 几乎所有其他的恶性涎腺肿瘤(eg, undifferentiated carcinoma未分化癌, squamous cell carcinoma鳞癌, mucoepidermoid carcinoma粘表, salivary duct carcinoma导管癌, adenoid cystic carcinoma腺样囊性癌, myoepithelial carcinoma肌上皮癌, and epithelial myoepithelial carcinoma上皮肌上皮癌).[26, 3, 18, 20, 11, 26, 27] The percentage of the malignant component varies widely; in some instances, the locations of the original benign pleomorphic adenoma are difficult to ascertain (see Media files 1-4).[28
- Author: M Sherif Said, MD, PhD; Chief Editor: M Sherif Said, MD, PhD
-
Carcinoma in situ
-
Intracapsular carcinoma
-
Minimally invasive (1.5 cm or less extension beyond the capsule)
-
Invasive/widely invasive (greater than 1.5 cm extension beyond the capsule)
Pathology of Carcinoma Ex Pleomorphic Adenoma
Different patterns of malignant change occur in pleomorphic adenoma, of which carcinoma ex pleomorphic adenoma is one form; the other 2 forms aretrue malignant mixed tumor (carcinosarcoma) and metastasizing pleomorphic adenoma.
carcinoma ex pleomorphic adenoma is defined as a carcinoma that arises in the epithelial and/or myoepithelial component of a pleomorphic adenoma. In most instances (75%), the luminal epithelial cells undergo malignant change. In 19% of cases, a dual epithelial/myoepithelial differentiated carcinoma is seen. Pure myoepithelial malignant change is seen in only 6% of cases.Carcinoma ex pleomorphic adenoma constitutes 99% of all cases of malignant mixed tumors; it develops in 6% of all pleomorphic adenomas. It constitutes 3.6-4% of all salivary gland tumors and 12% of all malignant salivary gland tumors.Carcinoma ex pleomorphic adenoma is seen in patients in the sixth to seventh decades of life.[2, 7] Gender preference has been variably reported in different series.[8, 7, 2] Carcinoma ex pleomorphic adenoma is rarely encountered in patients younger than 30 years and is rarely seen in children.[9, 10] The average median age at onset is 61 to 67 years; this median age at onset is 10 to 20 years older than the median age of patients with pleomorphic adenoma, lending support to the view that longstanding tumors are more prone to malignant change.
Etiology
Malignant transformation may occur up to 50 years after a pleomorphic adenoma is first diagnosed; the average period before malignant transformation is 20 years. The exact etiologic factors associated with malignant transformation are largely ill defined; however, exposure to radiation is thought to be a factor. It is also thought that malignant change may result from the development and accumulation of genetic instabilities within the tumor.[12]
A study by Hu et al suggests that promoter methylation of the p16 gene may be linked with the malignant evolution of pleomorphic adenoma to carcinoma ex pleomorphic adenoma. The increased expression in the cytoplasm of p16 protein combined with the decreased expression in the nucleus of this same protein may lead to this transformation.[13]
Interestingly, the rate of occurrence seems to increase with increases in the period during which pleomorphic adenoma is left untreated.[11] According to some investigators, the rate of malignant change is 1.5% in the first year in which the adenoma goes untreated; it increases to 9.5% after 15 years.
Location
Carcinoma ex pleomorphic adenoma more commonly occurs in the major salivary glands than in the minor salivary glands.
Carcinoma ex pleomorphic adenoma is most frequently seen in the parotid gland (67%); the submandibular gland is less frequently involved (15%). The sublingual gland is involved in only 1% of cases.[3, 4]
Fine-needle aspiration is generally one of the first steps taken in diagnosing a salivary gland mass. It is interesting to note that carcinoma ex pleomorphic adenoma tends to occur in the deep lobe of the parotid, in contrast to most pleomorphic adenomas, which tend to occur in the superficial lobe.[8] This fact may account for the low preoperative diagnostic accuracy and sensitivity of fine-needle aspiration in diagnosing carcinoma ex pleomorphic adenoma.
In the minor salivary glands, the palate is the most common site of occurrence.[14, 5, 15, 16, 17]
Clinical Features and Imaging
The patient usually presents with a history of a slowly growing, painless mass[18, 19] that suddenly or over a short period enlarges rapidly. Patients usually present with symptoms and signs suggesting malignancy (eg, fixation to surrounding structures,[11] occasional pain, skin infiltration, trismus,[8] facial nerve weakness, or palsy[20] ). Facial nerve weakness or palsy has been detected in approximately 23-40% of cases.[21, 22, 23]
Some patients with carcinoma ex pleomorphic adenoma have a history of multiple previous surgeries for pleomorphic adenoma[18] ; such a history might suggest the possibility of malignant transformation. This is relevant, considering the fact that some cases of very well-differentiated epithelial malignancy (eg, myoepithelial carcinoma ex pleomorphic adenoma) are sometimes not fully appreciated. Careful observation of the resected tumor may reveal malignancy.[24]
Gross Findings
Carcinoma ex pleomorphic adenomas vary considerably in size; sizes may range from 1 cm to greater than 20 cm. Macroscopic features that suggest malignant transformation in pleomorphic adenoma include poorly defined and/or infiltrative tumor margins,[3] the presence of foci of hemorrhage, and necrosis. However, some malignant tumors are well circumscribed[18, 25] (eg, noninvasive or minimally invasive varieties).
Microscopic Findings
The most common malignant epithelial subtype seen in carcinoma ex pleomorphic adenoma is poorly differentiated adenocarcinoma not otherwise specified (NOS). Almost all other malignant varieties of salivary gland tumors have been described (eg, undifferentiated carcinoma, squamous cell carcinoma, mucoepidermoid carcinoma, salivary duct carcinoma, adenoid cystic carcinoma, myoepithelial carcinoma, and epithelial myoepithelial carcinoma).[26, 3, 18, 20, 11, 26, 27] The percentage of the malignant component varies widely; in some instances, the locations of the original benign pleomorphic adenoma are difficult to ascertain (see Media files 1-4).[28]
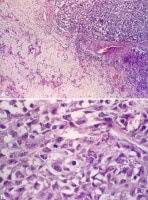 Undifferentiated carcinoma ex pleomorphic adenoma. Image A: In the right upper side of the photomicrograph, an undifferentiated carcinoma is seen infiltrating into the benign pleomorphic adenoma component, which appears on the left side of the photomicrograph. Image B: At higher magnification, the undifferentiated carcinoma shows pleomorphic tumor cells with focally vacuolated cytoplasm. A dark, star-shaped mitotic figure is readily apparent in the middle of the photomicrograph.
Undifferentiated carcinoma ex pleomorphic adenoma. Image A: In the right upper side of the photomicrograph, an undifferentiated carcinoma is seen infiltrating into the benign pleomorphic adenoma component, which appears on the left side of the photomicrograph. Image B: At higher magnification, the undifferentiated carcinoma shows pleomorphic tumor cells with focally vacuolated cytoplasm. A dark, star-shaped mitotic figure is readily apparent in the middle of the photomicrograph.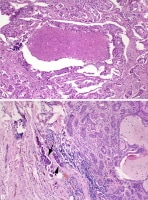 Salivary duct carcinoma ex pleomorphic adenoma. Image A: The malignant portion of the tumor is shown with the characteristic cribriform pattern and central comedo necrosis—characteristics usually seen in salivary duct carcinoma. Image B: The right side of the image shows a benign pleomorphic adenoma. Note the metastatic tumor emboli from the malignant component seen in Image A in the peritumoral vessels (arrows).
Salivary duct carcinoma ex pleomorphic adenoma. Image A: The malignant portion of the tumor is shown with the characteristic cribriform pattern and central comedo necrosis—characteristics usually seen in salivary duct carcinoma. Image B: The right side of the image shows a benign pleomorphic adenoma. Note the metastatic tumor emboli from the malignant component seen in Image A in the peritumoral vessels (arrows).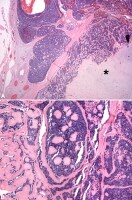 Adenoid cystic carcinoma ex pleomorphic adenoma. Image A: Adenoid cystic carcinoma surrounds and infiltrates the chondroid stroma (asterisk sign) of the adjacent benign pleomorphic adenoma. Note that the tumor appears to still be within the confines of the original tumor fibrous capsule surrounding the circumference. Image B: At higher magnification, the characteristic cribriform (Swiss cheese) pattern of adenoid cystic carcinoma with intervening reduplicated and hyalinized basal lamina is apparent.
Adenoid cystic carcinoma ex pleomorphic adenoma. Image A: Adenoid cystic carcinoma surrounds and infiltrates the chondroid stroma (asterisk sign) of the adjacent benign pleomorphic adenoma. Note that the tumor appears to still be within the confines of the original tumor fibrous capsule surrounding the circumference. Image B: At higher magnification, the characteristic cribriform (Swiss cheese) pattern of adenoid cystic carcinoma with intervening reduplicated and hyalinized basal lamina is apparent.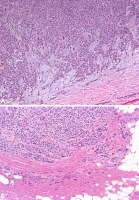 Myoepithelial carcinoma ex pleomorphic adenoma. Image A: The tumor is infiltrating the myxoid stroma of pleomorphic adenoma and almost completely replaces the substance of the benign tumor. Note the bland nature of the tumor cells in this malignancy; this may cause difficulties in recognizing malignancy in small tissue biopsy specimens, particularly in light of the fact that myoepithelial cells are a constituent of pleomorphic adenoma cell populations. Image B: The malignant nature of the tumor is obvious in this image. Note how the tumor extends into the surrounding tissue, infiltrating it in cords and in single cell files.
Myoepithelial carcinoma ex pleomorphic adenoma. Image A: The tumor is infiltrating the myxoid stroma of pleomorphic adenoma and almost completely replaces the substance of the benign tumor. Note the bland nature of the tumor cells in this malignancy; this may cause difficulties in recognizing malignancy in small tissue biopsy specimens, particularly in light of the fact that myoepithelial cells are a constituent of pleomorphic adenoma cell populations. Image B: The malignant nature of the tumor is obvious in this image. Note how the tumor extends into the surrounding tissue, infiltrating it in cords and in single cell files.
Although in most instances, the features of malignant transformation are evident, some very well-differentiated cancers (eg, myoepithelial carcinoma) or cancers that exhibit morphologically limited nuclear atypia may be somewhat challenging to evaluate for the presence of malignancy.[24]
Clinical experience has engendered an appreciation of the evolution of carcinoma ex pleomorphic adenoma over time. Presentations vary and may include the following: carcinoma in situ, in which atypical nuclei replace the ductal luminal layer while the not atypical myoepithelial layer is retained focally or multifocally[29, 30, 31] ; carcinoma that breaks from the confines of the myoepithelial layer to invade the surrounding stroma while remaining intracapsular; and frankly invasive cancer that extends beyond the capsule of the pleomorphic adenoma.[18, 32]
Tumors that invade beyond the capsule into the surrounding tissue by less than 1.5 cm are considered minimally invasive; overall, patients with this form of disease have an excellent prognosis. Those tumors that invade beyond 1.5 cm are considered invasive; with such tumors, prognoses vary in accordance with tumor stage and grade.[27] Tumors should be carefully evaluated with regard to the degree of invasion of tumor, because treatment will vary accordingly.
The grade of the cells seen in the cancerous epithelial component varies from well differentiated to poorly differentiated to anaplastic, depending on the cancerous subtype. Most carcinomas are high grade, but some are low grade. However, the histologic grades of the tumor should be judged according to how the carcinomatous category is usually graded in an individual tumor. For example, a mucoepidermoid carcinoma ex pleomorphic adenoma is histologically categorized as being of low, intermediate, or high grade histologically, as is done in the case of a mucoepidermoid carcinoma occurring as an individual tumor.
Perineural invasion[18] and angiovascular invasion are commonly encountered in the invasive types; necrosis is prominent in the high-grade types
mmunohistochemistry
Determining the different types of cells in the tumor by use of immunohistochemistry may aid in establishing an accurate diagnosis Tumor markers are described in the eMedicine article Pleomorphic Adenoma. However, specific additional markers have occasionally proven useful in cases of carcinoma ex pleomorphic adenoma. Immunoreactivity for p53 was observed by some authors to be a useful tool in differentiating pleomorphic adenoma from carcinoma ex pleomorphic adenoma. Indeed, some authors have reported that p53 may be used as a means of early detection of malignant change in pleomorphic adenoma[33, 34, 35, 29, 36] ; other authors, however, have disputed this claim.
Immunoreactivity (overexpression) for HER2 (c-erbB-2) has been used by some investigators to differentiate carcinoma ex pleomorphic adenoma from atypical pleomorphic adenoma[36, 37, 38, 39] ; others have found no significant correlation. Immunoreactivity for cyclin A and its increased expression have also been noted by some investigators to correlate with the development of carcinoma ex pleomorphic adenoma.[40]
Recently, expression of X-linked inhibitor of apoptosis protein (XIAP) (a protein that is associated with aggressive behavior in tumors) was studied in both pleomorphic adenoma and carcinoma ex pleomorphic adenoma.[41] The results indicated an increase in the expression of XIAP from pleomorphic adenoma to cellular pleomorphic adenoma to carcinoma ex pleomorphic adenoma, or even atypical areas within pleomorphic adenoma. Such findings suggest that XIAP expression may be an additional marker to aid in the evaluation of malignant change in pleomorphic adenoma.
Molecular/Genetics
Rearrangements of chromosome 8q12 and alterations in chromosome 12q13-15 with amplification of HMGIC, HMGA2 and MDM2 genes are frequent and may contribute to malignant transformation.[42, 43]
Deletions of chromosome 5 have been reported, as have alterations in chromosome 17q and 6q deletion.
p53 alterations are seen in 29-75% of cases, and p53 overexpression is seen in 41-75% of cases, suggesting that p53 plays a role in the development of carcinoma ex pleomorphic adenoma.[36]
HER2/neu (c-erbB-2) overexpression or gene amplification occurs in 21-82% of cases.[36, 39] The role HER2/neu remains controversial.[36]
Tumor Spread and Staging
The American Joint Committee on Cancer (AJCC)[44] staging system for malignant tumors of the major salivary glands (ie, the parotid, submandibular, sublingual glands) is as follows:
Table. TNM classification (Open Table in a new window)
| T- | Primary tumor |
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor 2 cm or less in greatest dimension without extraparenchymal extension |
| T2 | Tumor more than 2 cm but not more than 4 cm in greatest dimension without extraparenchymal extension |
| T3 | Tumor more than 4 cm and/or tumor having extraparenchymal extension |
| T4a | Tumor invades skin, mandible, ear canal, and/or facial nerve |
| T4b | Tumor invades skull base and/or pterygoid plates and/or carotid artery |
| N- | Regional lymph nodes |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis into a single ipsilateral lymph node, 3 cm or less in greatest dimension |
| N2 | Metastasis into a single ipsilateral lymph node, greater than 3 cm in greatest dimension but not greater than 6 cm in greatest dimension, or in multiple ipsilateral lymph nodes, none greater than 6 cm in greatest dimension, or in bilateral or contralateral lymph nodes, none greater than 6 cm in greatest dimension |
| N3 | Metastasis in a lymph node greater than 6 cm in greatest dimension |
| M- | Distant metastasis |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
Table. Stage grouping (Open Table in a new window)
| Stage | Grouping |
| Stage I | T1 N0 M0 |
| Stage II | T2 N0 M0 |
| Stage III | T3 N0 M0 |
| T1 N1 M0 | |
| T2 N1 M0 | |
| T3 N1 M0 | |
| Stage IVA | T4a N0 M0 |
| T4a N1 M0 | |
| T1 N2 M0 | |
| T2 N2 M0 | |
| T3 N2 M0 | |
| T4a N2 M0 | |
| Stage IVB | T4b Any N M0 |
| Any T N3 M0 | |
| Stage IVC | Any T Any N M1 |
Tumor spread can occur by direct extension and lymphovascular or perineural invasion. As mentioned above, the tumor can be histologically divided on the basis of temporal evolution into the following:
For carcinoma ex pleomorphic adenoma arising in the minor salivary glands, staging is determined in accordance with the system of the region of occurrence.
Prognosis and Predictive Factors
Patients with noninvasive and minimally invasive tumors have an excellent prognosis; for these tumors, the metastatic potential is very low.[45, 32]Tumors in the invasive category tend to behave in a more aggressive fashion; up to 25-50% of patients experience recurrence,[3, 7, 18] and up to 60-70% of patients develop local or distant metastasis.[3] Metastatic sites include lymph nodes, bone (especially vertebral bodies), and the brain.
The stage of the disease plays an important role in prognosis. Patients with stage I disease generally have an excellent prognosis, whereas the survival rate for patients with stage IV disease is extremely poor. A recent study of 22 patients with CXPA confirmed this general tendency.[21] In that study, both the 5-year disease-specific and overall survival rates were 60%; the recurrence-free survival rate after 5 years was 85%. Interestingly, in this series, the incidence of higher-stage disease correlated with older age (the average age of patients with stage IV disease was 67 years), whereas lower-stage disease correlated with younger age (the average age of patients with stage I or II disease was 53 years).
The survival rate has been correlated with the size, type, and histologic grade of cancer, particularly with regard to the widely invasive types. Patients with undifferentiated carcinoma have the worst survival rate (30%); those with polymorphous low-grade adenocarcinoma have the highest survival rate (96%).[26]