| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- 系统学病理及英语病理学之二
The cellular elements of the central nervous system are neurons and glia. All of these cells have processes in addition to their cell bodies. The neuronal processes are called axons and dendrites. In the peripheral nervous system, there are no glia. There are Schwann cells which surround the axons and produce myelin in the same manner as the oligodendroglia of the CNS. Interestingly, the Schwann cells also become phagocytes, devouring the debris from injured peripheral nerves, and this property is not shared by the oligodendroglia.
PATHOLOGIC CHANGES IN NEURONAL CELL BODY
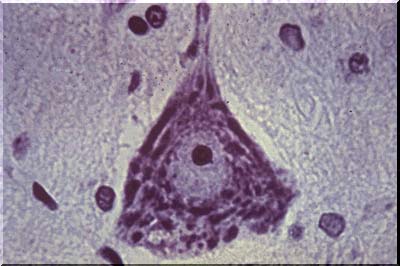
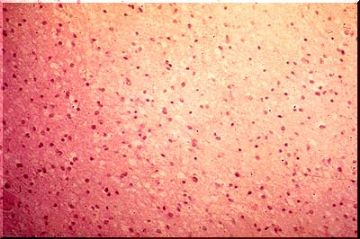
The image above is an example of a normal anterior horn nerve cell. The normal anterior horn cell serves as a good illustration of a normal neuron. The nucleus is centrally placed and contains a large, prominent nucleolus. In the cytoplasm, large clumps of blue-black material are seen which represent the prominent aggregates of ribonucleoprotein (RNA). This material is often called Nissl substance, after the man who devised special stains for staining it. One such stain, cresyl violet, has been used in this image.
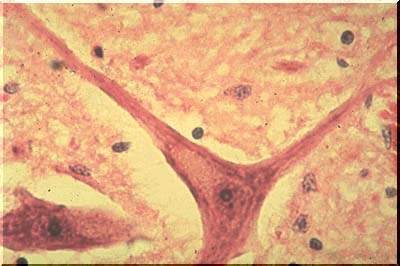
The image above, with the more routine hematoxylin and eosin stain, also discloses Nissl substance, which is a shade of purple. Although the neurons illustrated in this image are typical of large neurons, please remember that neurons of all sizes exist in the central nervous system.
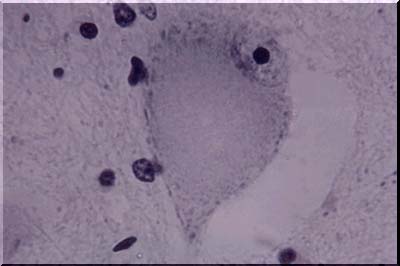
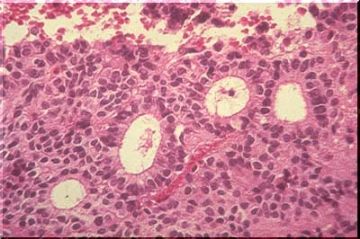
The image above illustrates central chromatolysis. In response to transection or destruction of the axons, whether by mechanical trauma or by other means, a characteristic change known as central chromatolysis occurs in the neuronal cell body. The nucleus moves to an eccentric position, the Nissl material is visible only peripherally, and the central area of the neuron is free of stainable material. This is a reversible change and electron microscopy shows that the ribonucleoprotein is dispersed rather than aggregated as in the normal neuron. The normal appearance of many neurons, especially in the brain stem, resembles that of central chromatolysis. The reversible movement of RNA within the cell body in response to axonal injury is apparently related to the call on the cell for increased protein synthesis - a demand arising as the cell attempts to regenerate a new axon. Regeneration can be completed in the peripheral nervous system, but is only abortive in the central nervous system. When the demand for increased protein synthesis is ended, the Nissl substance returns to its normal position. When the axon is injured very close to the cell body, or in instances of direct injury to the cell body, whatever the cause, the cell body may be irreversibly damaged and simply disappear. Disappearance is a frequent end result of ischemic and/or anoxic damage. Prior to disappearance, the cell body may show vascular degeneration or become surrounded by or covered by microglial cells (neuronophagia).
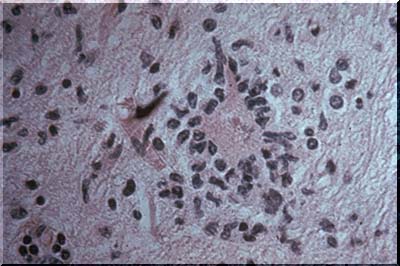
The image above is an example of neuronophagia. Neuronophagia is very common after viral infection of the CNS, but its occurrence is not restricted to viral diseases. The stain is H&E so the microglia are represented only by elongate , nuclei stained with hematoxylin.
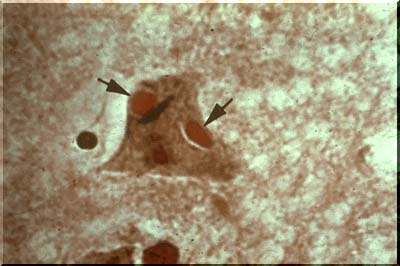
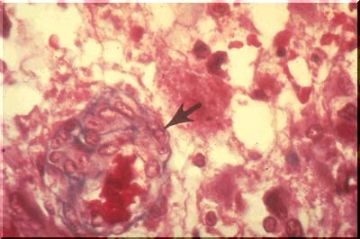
Viral infection is often accompanied by the presence of inclusion bodies, either within the nucleus or the cytoplasm of the neuron. In this case the patient had rabies and the inclusions are called Negri bodies.Thesel cytoplasmic inclusions are illustrated by the red ovoids as seen in the image above (arrows).
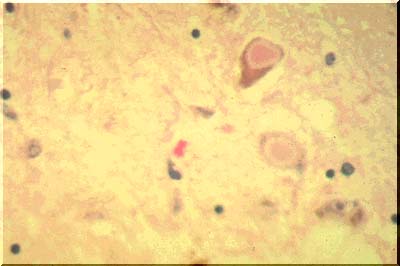
Cytoplasmic inclusions are also characteristic of Parkinson's disease. They are called Lewy bodies. The large pink bodies seen here (image above) are illustrative of this condition. The pink centers of the inclusion bodies contain several substances including synuclein. The latter is a normally found at synapses and its function is unknown.
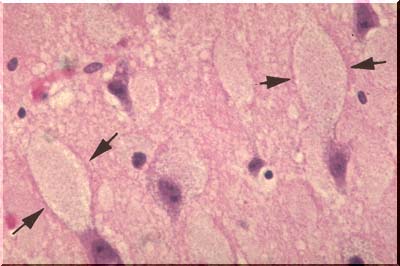
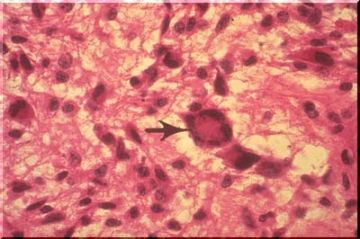
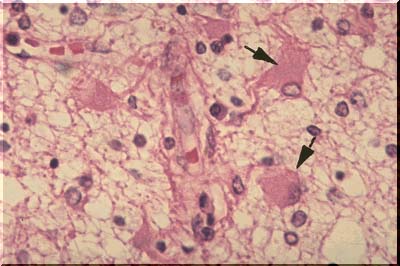
In storage disease, the neuronal cell body may become tremendously distended by the storage product. Some of the distended cell bodies on the image above are demarcated by arrows. When treated with special silver stains, elongate black neurofibrils can be demonstrated within the normal neurons as illustrated on this image. They extend down the entire length of the axon. The function of the fibrils is not known, but they may represent artifactually clumped tubules which, in turn, (hypothesis), may serve as conduits for the movement of intracellular materials from the cell body, or factory, down the axon to the synapse.
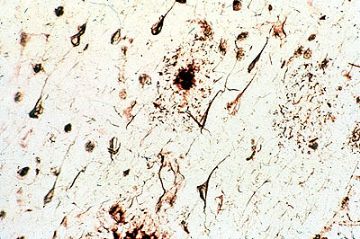
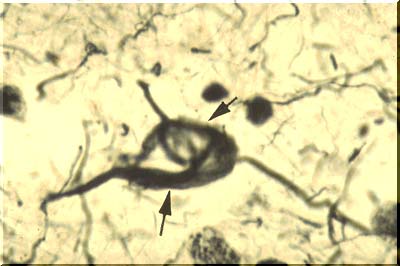
As we age, most of us will develop alterations of neurofibrils in at least some of our neurons. They will become clumped and twisted into odd shapes like tennis rackets or skeins of wool. In Alzheimer-type dementia, tremendous numbers of these neurofibrillary tangles are seen. In this image, arrows point to a neuron filled with such a tangle. Perhaps this leads to interruption of transport down the axon and this in turn is related to deteriorating intellectual function.
At the same time, the dendrites may degenerate to produce oval, haystack-like masses of silver-stained (argyrophilic) fibers. These masses are known as senile plaques or neuritic plaques, the adjective signifying the fact that the plaque is composed of degenerated "neurites".Another change accompanying aging is an increase in the amount of yellow-brown lipofuscin pigment in the neuronal cytoplasm.In addition to lipofuscin, some neurons contain neuromelanin. This material is an end product of catecholamine metabolism and is found in the neurons of the substantia nigra (images below), imparting a black appearance to this structure when seen in a sliced midbrain and presenting as dark brown granules in the neurons observed under the microscope.
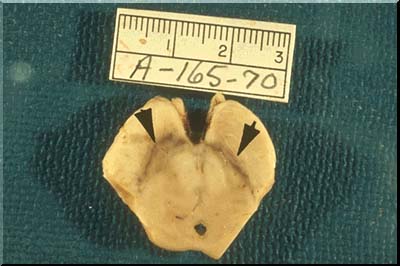
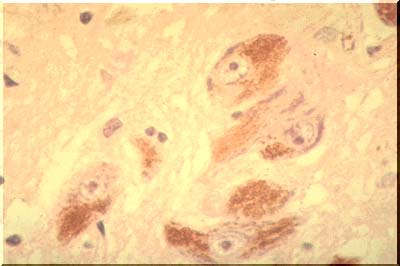
The neurons of the substantia nigra degenerate and disappear in Parkinson's disease. Their pigment is phagocytosed by macrophages which carry it away. The substantia nigra then becomes pale, a morphologic tombstone representing a disease with disrupted catecholamine synthesis.
PATHOLOGIC CHANGES IN AXON
We will now progress to a discussion of injuries involving the extension of the cell body known as the axon. When the axon is severed or irreversibly injured, all of the axon degenerates distal to the site of injury. The entire axon degenerates at once, as does its myelin sheath. This form of degeneration is called Wallerian degeneration, after Waller, the man who first described it. Axonal injury is readily manifest by special silver stains and is indicated by axonal swelling, disintegration, and finally, disappearance. Myelin stains reveal degeneration and then loss of myelin all along the affected axon. Wallerian degeneration can occur in either the central nervous system or in the peripheral nerves. During the process of myelin degeneration in peripheral nerves, phagocytes engulf the myelin debris. Most of these phagocytes come from monocytes; some come from Schwann cells. You will remember that the Schwann cell is also the cell which has wrapped around the axon of the peripheral nerve to form the myelin. The analagous cell of the central nervous system is the oligodendroglia. Unlike the Schwann cell, the oligo does not become a phagocyte when myelin breaks down. Instead, in the central nervous system, phagocytosis of myelin debris is performed by mesodermal elements, like monocytes entering from the blood. In the peripheral nervous system, axons can successfully regenerate after Wallerian degeneration. In the CNS, regenerative sprouts may appear but will fail to continue growth and/or to make renewed functional connections with their original targets.
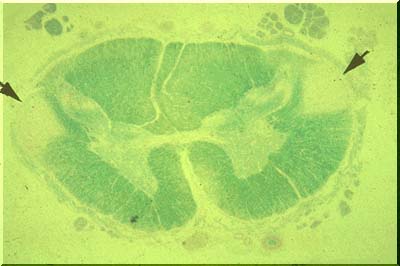
When Wallerian degeneration occurs in a large number of axons, running together in a compact "tract," tract degeneration is readily demonstrated on myelin stains. The image above displays a spinal cord stained with Luxol fast blue. Pallor of the lateral columns (pyramidal tracts) indicates lack of myelin in these columns or tracts. Wallerian degeneration has occurred.
In addition to Wallerian degeneration axons may undergo a different kind of breakdown. This is called "distal axonopathy" or "dying back". Here, for unknown reasons, the portions of the axon farthest from the cell body begin to die first. Then death proceeds up the axon, toward the cell body. The myelin will also degenerate along the affected segments of the axon. The changes occur in patchy fashion. They may be seen in the central nervous system or in the peripheral nervous syste. In the latter several metabolic diseases may cause dying back.
-
本帖最后由 于 2010-06-21 08:05:00 编辑

- 赚点散碎银子养家,乐呵呵的穿衣吃饭
INTRODUCTION
There are three types of glia: astrocytes, oligodendroglia and microglia. Astrocytes and oligodendroglia are neuroectodermal derivatives. The astrocyte is the principle cell responding in a non-specific way to injuries of the nervous system. A major function of the oligodendroglia is to produce myelin. Microglia are members of the mononuclear phagocyte system (formerly called Reticulo Endothelial System). There is a fixed population which comes from bone marrow and seed the brain during fetal life. Additional monocytes enter the brain from the blood especially after various destructive insults. Generally the tissue macrophages come from these monocytes. The latter may present antigens. In some situations the fixed population of microglia can become macrophages. In addition to becoming macrophages there are other functions of microglia, e.g., cytokine release, antigen presentation.
In the peripheral nervous system there are no glia. There are Schwann cells whose nature was discussed in the chapter on the neuron and its processes . They are mentioned only to remind you that they share one property in common with oligodendroglia, namely the production of myelin.
The reactions of astrocytes, described in this chapter, occur over and over again in different disease settings. Thus, after reviewing this chapter, the student should be prepared to approach other chapters dealing with specific disease entities.
ASTROCYTES
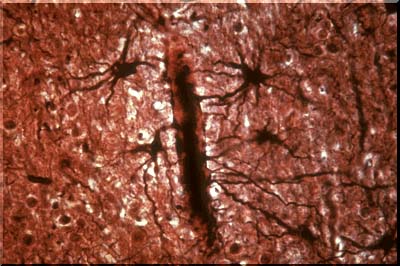
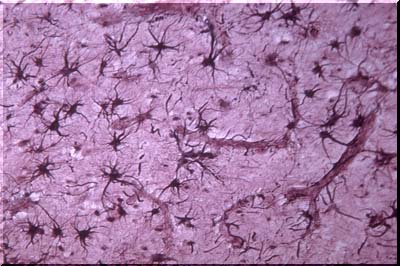
This image illustrates several normal astrocytes stained with a special gold stain named after Cajal. This and other special stains disclose the starfish like processes of the normal astrocyte. Often, as is the case of several astrocytes in this image, the processes attach to very small blood vessels. Until recently it was thought that these foot process attached to capillaries. Now it has been shown that they do not, but attach instead to precapillary arterioles and post capillary venules. These processes are called foot processes or "sucker feet." The latter term implied that the foot processes serve as conduits for substances from the capillary lumen to the brain. In fact, this does NOT occur. Transport is through the capillary walls into the extracellular space. The true capillaries are wrapped by very fine astrocytic processes. The role of these fine wrappings in determining or controlling the passage of materials through the capillary wall or through the tight junctions between capillary endothelial cells is unclear.
If astrocytes do not act as conduits, what do they do? Their known and hypothesized functions are continually being expanded. They include: removal of potassium ion from vicinity of firing neurons; removal of glutamate, the principal excitatory transmitter, from vicinity of firing neurons; metabolism of glutamate to lactate which is then liberated from the astrocyte and may serve as partial energy source for neurons;synthesis of glutamine for transport to neurons which convert it to glutamate; production of diverse cytokines with diverse purposes; release of molecules that signal nearby vessels to express and translate structural and functional proteins required to produce and maintain barriers to proteins and other solutes [i.e. these make up a variety of so-called "blood brain barriers"]; release of dilators and constrictors of arterioles which may help couple the local vascular supply to the demand of nearby neurons.
Not all astrocytes have long slender processes. Some, predominantly in grey matter, have shorter processes with more frequent thorn-like side branches. Considerable confusion has arisen because the latter type of astrocyte has been called "protoplasmic" while the former has been called "fibrous." These terms do not refer to the shape of the cell body or its processes. They refer instead to the presence, or relative absence of delicate fibrils within the cell body and processes. These are best seen with a different stain, phosphotungstic acid hematoxylin (PTAH). Normal fibrous astrocytes have large numbers of intracytoplasmic fibrils; normal protoplasmic astrocytes do not. The intracytoplasmic fibrils may represent bundles of intermediate filaments. In any case, such filaments, seen with the electron microscope, are characteristic of normal astrocytes, are more prevalent in fibrous astrocytes, increase in number when astrocytes react to injury, and contain an epitope that is stained by an antibody to glial fibrillary acid protein (GFAP). This antibody labels astrocytic cytoplasm for light microscopy.
Now you may wonder why we have emphasized special stains for astrocytes. That is because when astrocytes are normal, their cytoplasm does not stain with ordinary stains like hematoxylin and eosin. Only the nucleus stains, and, in fact, the same is true for the other two types of glia. Thus, the normal astrocyte is recognized on routine stains by its oval, vesicular nucleus, while the oligodendroglia is distinguished by its smaller, more perfectly round, and very darkly staining nucleus. Some of the latter are indicated by arrows on the image shown later in this chapter.. The microglia has a small elongated or cigar-shaped nucleus. Now let us review the features and nomenclature of normal astrocytes:
Astrocytes respond dramatically in response to injury of the nervous tissue. Astrocytic reaction is the most important evidence that "something is wrong" with the brain or cord. Astrocytes respond to injury by (1) multiplying, (2) increasing the length of their processes, and (3) changing their staining characteristics so that their cytoplasm, normally unstained by H&E, now becomes eosinophilic. Not all of these changes need occur. When the cytoplasm does become stainable with eosin, the nucleus is often displaced to the periphery and the cell looks plump or fat. Such a cell is often called gemistocytic or a gemistocyte from a German word implying "stuffed" as in after eating.. Do not apply the term protoplasmic to these plump, reactive astrocytes. Remember that the term "protoplasmic" is reserved for normal astrocytes with few intracytoplasmic fibrils. In fact, both protoplasmic astrocytes and fibrous astrocytes can react to tissue injury. When they do so, they both may show an increase in intracytoplasmic fibrils.
THE FIGURE BELOW SHOWS REACTIVE ASTROCYTES STAINED WITH H&E. NOTE VESICULAR NUCLEI AND PINK CYTOPLASM. THE PINK MESH BETWEEN CELL BODIES IS REALLY PART OF THE CELL AND REPRESENTS CELL PROCESSES.
THE FIGURE SHOWS GOLD STAINED REACTIVE ASTROCYTES WHICH ARE INCREASED IN NUMBER COMPARED TO NORMAL.
THE FIGURE BELOW SHOWS REACTIVE ASTROCYTOSIS WITH THE PTAH STAIN. THE PROCESSES ARE BLUE.
This image shows the edge of a so called glial "scar." It is a dense tangle of delicate astrocytic processes stained, in this case, by PTAH. Please remember this so called "scar" does not consist of collagen, and unlike collagen, which is an extracellular material produced by fibroblasts, the glial processes or fibers are cytoplasmic extensions of the cells themselves. Also note that when CNS tissue dies, dense glial scars like that shown here, rarely fill in the resulting defect. Instead, the defect may remain a cyst, or contain only a loose mesh of glial fibers.

In hepatic failure, astrocytes proliferate without developing eosinophilia. Known as Alzheimer Type II astrocytes, they are characterized by irregularly shaped nuclei with exaggerated vesicular appearance. They may reflect the importance of astrocytes in ammonia metabolism. Ammonia is elevated in liver failure and can elicit formation of these astrocytes.
OLIGODENDROGLIA
Oligodendroglia are glial cells with few processes, hence the prefix "oligo." These processes may wrap around axons to form myelin like the Schwann cell of the peripheral nervous system. Thus, in diseases characterized by myelin loss, there may be a great diminution in the numbers of oligoglia. This will be especially noticeable in white matter as opposed to grey, since in the latter axons and their myelin sheath are normally separated by cell bodies of neighboring neurons and glia, while in the white matter there are no neuronal cell bodies, so that the myelinated axons form compact bundles which normally stain quite intensely.
THE ARROWS POINT TO OLIGODENDROGLIAL NUCLEI. CYTOPLASM REMAINS UNSTAINED WITH H&E 
MACROPHAGES AND MICROGLIA
The macrophage in the CNS looks like the round, foamy, or vacuolated macrophage found in any organ. In the CNS, the macrophage is sometimes called a "Gitter" cell. In many conditions these macrophages come from circulating monocytes. This is particularly true in destructive lesions of the brain such as traumatic lesions or in infarction. However in some circumstances macrophages may develop from resident microglia. Microglia are "cousins" of the macrophage/monocyte and share some CD sites with them.
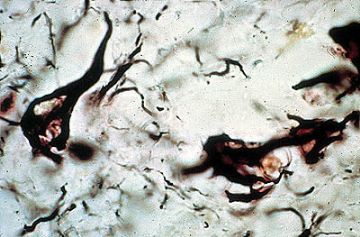
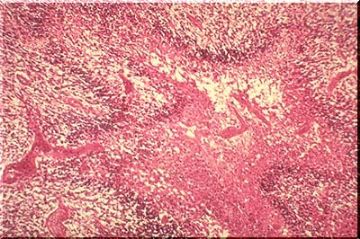
Microglia can be stained with a special silver stain and with antibodies directed against some lectins. . They arrive in the brain from bone marrow progenitors either during fetal/neonatal life or later. Thus, they represent a resident population of reticulo endothelial (mesenchymal) cells within the CNS. They may present antigen, release cytokines and have other functions identical to that of related RES cells in other organs. Sometimes the microglia simply proliferate and their cell bodies elongate. They are then called "rod cells". One form of tertiary syphillis--so called "general paresis" or "paralysis of the insane"-- is characterized by huge numbers of these rod cells throughout the brain. A few of these rod cells are illustrated in the silver stained image shown below. 
THE FIGURE SHOWS MICROGLIA STAINED WITH THE HORTEGA STAIN [SILVER CARBONATE]. THESE MICROGLIA HAVE THE ROD-LIKE FORM AND ARE SOMETIMES CALLED ROD CELLS. THEY CAN ALSO BE STAINED WITH A STAIN DIRECTED AGAINST ONE OF THE LECTINS.
THE FIGURE BELOW IS A LOW POWER VIEW OF A BRAIN AFFLICTED WITH TERTIARY SYPHILIS AND SHOWS THE HUGE NUMBER OF PROLIFERATING ROD CELLS THAT MAY BE SEEN.

- 赚点散碎银子养家,乐呵呵的穿衣吃饭
Alzheimer's Disease
This is a section of the hippocampus impregnated with silver (Bielschowsky) for demonstrating two specific changes: a) senile plaques that appear as small collections of dark, irregular, thread-like structures often with a brownish material in the center. The central core is represented by amyloid and the irregular, beaded linear structures represent abnormal neurites (small dendrites and axons with degenerative changes). b) neurofibrillary tangles--dark staining fibrillary material within neurons. They often occupy the entire neuron and appear flame-shaped. The fine structure of these fibrils is characterized by the presence of constrictions at regular intervals. Both senile plaques and neurofibrillary tangles are abundant in Alzheimer's disease although they are seen in smaller number in elderly individuals with dementia.
Slide 15.1
Typical senile plaques with central core and degenerating swollen neurites around it. Note many neurofibrillary tangles.
Slide 15.2
Higher magnification of Alzheimer neurofibrillary tangles.

- 赚点散碎银子养家,乐呵呵的穿衣吃饭
Meningioma
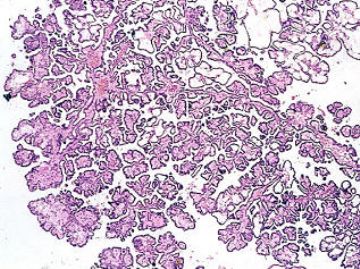
Meningiomas are derived from arachnoidal cells. The histology of these tumors is quite variable. The tumor in some of them is composed of meningeothelial cells. Some other tumors are composed of elongated cells and are called fibroblastic meningioma. Sometimes both cellular elements are present in the tumor. Two characteristic features of most meningiomas are: a) whorl formation b) psammoma body. In general these tumors are benign.
Slide 17.1
This shows the gross appearance of a convexity meningioma attached to the dural leaflet and indenting the underlying brain.
Slide 17.2
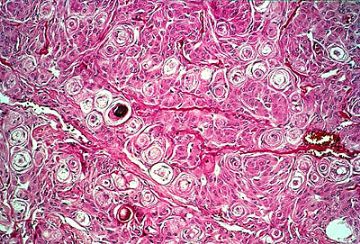
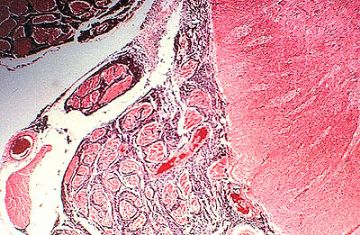
Low power view of a typical meningioma showing whorl formation and psammoma body.
Slide 17.3
Higher magnification of whorls and psammoma body.

- 赚点散碎银子养家,乐呵呵的穿衣吃饭
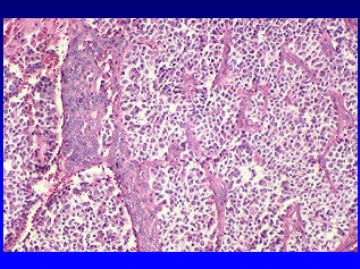
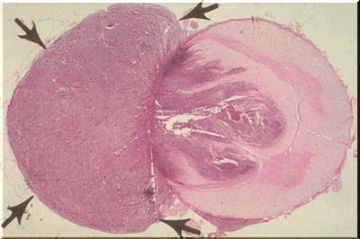
Medulloblastoma
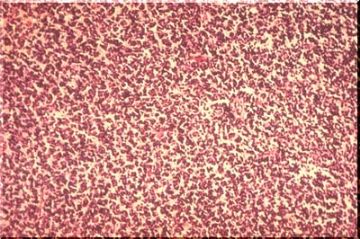
This is a malignant tumor in children that arises in the cerebellum usually in the vermis. The histology of this tumor is characterized by extreme cellularity and little stroma. The tumor cells are primitive neuroectodermal in nature and may differentiate to both neuronal and glial elements. These tumors grow into the fourth ventricle causing obstructive hydrocephalus and also frequently spread along the CSF pathways.
Slide 25.1
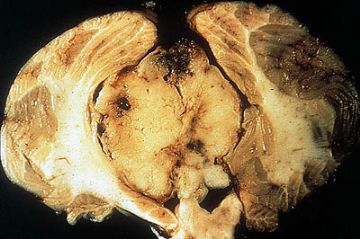
Gross appearance of a medulloblastoma arising from the cerebellum and growing into the fourth ventricle
Slide 25.2
Low power view of the spinal cord showing subarachnoid dissemination of medulloblastoma. The spinal roots are diffusely infiltrated by tumor cells.
Slide 25.3
The individual tumor cells are small, ovoid or slightly elongated with high nuclear cytoplasmic ratio. Except for an occasional blood vessel, no stroma is seen.

- 赚点散碎银子养家,乐呵呵的穿衣吃饭
- Glioblastoma multiform 55%
- Astrocytoma 20%
- Ependymoma 6%
- Oligodendroglioma 5%
- Medulloblastoma 6%
- Other 8%
Tumors of Brain Parenchymal Cells
Four cell types (astrocytes, oligodendroglia, ependymal cells, neurons) or their recognizable precursors give rise to virtually all tumors of primary brain tissue. However, very primitive, undifferentiated and difficult to classify tumors are sometimes encountered.
Generally speaking, the more primitive the appearance of the tumor cells, the more rapidly the tumor will grow. You will be shown the histology of the more important tumors to demonstrate the point that the neoplastic cells mimic or form caricatures of the cell of origin, either in its normal, reactive, or immature state (this is what really permits one to make correct diagnoses), and to help you understand the systems of grading malignant brain tumors.
GRADING SYSTEM
At present ,most pathologists use the World Health Organization system for classifying and grading tumors. In general the principles underlying WHO are similar to those employed years ago by Kernohan.
On the I-IV scale that was devised by Kernohan the highest grades indicate a greater degree of malignant change (ditto WHO). These malignant changes include increased cellularity; pleomorphism of cells, including bizarre and giant forms; hyperchromasia of nuclei; presence of immature cells; vascular proliferation and necrosis. These malignant changes are seen much more frequently in the astrocytoma series than in other neuroectodermal tumors and the discussion of anaplastic changes in astrocytomas will serve as a prototype for the others.
Others use a three level grading system whose criteria of ascending grade are similar to the 4 point system but with obvious compression of two grades into one. Instead of grading, some pathologists have used terms that stress resemblance of neoplastic cells to normal cells at various stages of maturity. This schema would use, in the astrocytic series, for example, the terms astrocytoma, anaplastic astrocytoma and glioblastoma multiforme to denote increasing malignancy [i.e. decreasing maturity of cells]. It is important, irrespective of the system used, that the pathologist and the clinician understand the pathologists' terms in the way that the pathologist intended with respect to prognosis.
Progress in genetic research will undoubtedly lead to further refinements in grading brain tumors. For example, glioblastomas have been found to be of two types, one with a P53 mutation and one with amplification of epidermal growth factor receptor. The former appears to arise from a more differentiated astrocytoma; the latter arises denovo (i.e., not in a preexisting tumor).
Moreover, some oligodendrogliomas in adults--but not in children--have been found to have deletions [ loss of heterozygosity] on chromosome 1 and 19. These having better prognosis and differential responsivity to some chemotherapeautic agents
INCIDENCE
The incidence in percentages of the more important tumors of brain parenchyma are:
Total: 100%
Astrocytomas are largely tumors of adults but are still the leading primary brain tumor in children. Including glioblastoma multiforme [grade 4 astrocytoma], they account for about 75% of all of tumors arising from brain parenchyma.
These tumors can be found anywhere in the CNS, but are most frequently seen in the anterior half of the cerebrum in adults or in the posterior fossa (cerebellum and brainstem) and hypothalamus in children (juvenile type). Some of the more slowly growing astrocytomas (grade I-II) may permit survival for several years, whereas patients with glioblastoma multiform (grade IV astrocytoma) or "anaplastic" astrocytoma (grade III) usually die within a year following surgical decompression of the tumor.
However, since astrocytomas are virtually always invasive and display uncontrolled growth, even grade I tumors (sometimes referred to as "benign") may be truly malignant. Some grade one tumors appear to really be benign, demonstrating a sort of encapsulation by dense astrocytic tissue (?benign reactive astrocytosis) and can be "shelled out" by the neurosurgeon. These are often "pilocytic" astrocytomas--see later--and sometimes are cystic (in the cerebellum). The higher grade (III-IV) tumor is seen most frequently in middle aged adults and unfortunately accounts for 55% of all neuroectodermal tumors.
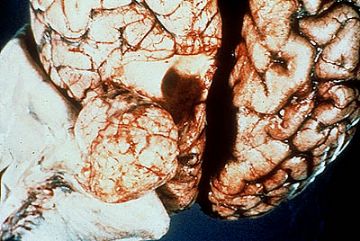
Grossly, there is poor demarcation of astrocytomas from the surrounding parenchyma. It is difficult to define where the tumor ends and normal tissue begins. All that may be seen is enlargement of the involved portion of brain and loss of distinction between grey and white matter. However, higher grade astrocytomas are usually strikingly visible since they may show marked necrosis and discoloration like the glioblastoma of the frontal lobe in the image below.
Note that the tumor is infiltrating the corpus callosum and crossing over to the opposite side of the brain. Tumor cells will be seen microscopically, even in the areas about the tumor which appear grossly normal.
We will begin by discussing the first or slow growing group (grade I). Here, the only unusual feature encountered may be an increase in cellularity. In the image below there are too many astrocytes for normal brain tissue, but the cells themselves have the appearance of normal astrocytes with uniform, oval, vesicular nuclei and fibrillar cell processes (a "fibrous" or "fibrillary" astrocytoma).
Another type of slow growing astrocytoma is the protoplasmic astrocytoma, in which cystic degeneration of the cytoplasm is present and the processes are less prominent in this image.
A unique variety of this tumor is restricted to a mural nodule in a cystic cavity (cystic cerebellar astrocytoma). See image below
In that case, it can often be cured by excision, a feat which is rarely possible with neuroectodermal tumors. In fact, this astrocytoma may be truly benign in that it does not invade surrounding tissue. In any case, it is often characterized by very elongated, neoplastic astrocytes, which are called piloid because they look like hair. Piloid astrycytomas may also be found in other areas, including the brain stem. Here, too, they have very slow growth and would be theoretically totally removable except for their location, which would make such surgery too dangerous.
The cystic cerebellar astrocytoma should not be confused with hemangioblastoma, another benign cystic tumor of the cerebellum. See section 1 of the 4 tumor sections in this chapter for information about that tumor.
Piloid astrocytomas are characterized by Rosenthal fibers which are worm-like eosinophilic bundles with irregular profiles. These are really clumped astrocytic processes with their intracellular thin filaments. Oval granular eosinophilic bodies are also seen. Both types of structure are non-specific findings indicative of long-standing pathological processes as would be the case in a benign tumor. Rosenthal fibers are also characteristic of a rare leukodystrophy--Alexander's disease--in which abnormalities have been found on the gene coding for glial fibrillary acidic protein.
The image below displays the brightly eosinophilic Rosenthal fibers and eosinophilic bodies in a slow growing astrocytoma.
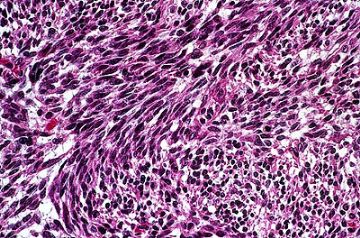
Now we will discuss the features which indicate rapid growth potential in astrocytomas, grade III-IV, respectively named by most neuropathologists as anaplastic astrocytoma and glioblastoma multiforme. The most important criterion will be the appearance of the nucleus. Atypical nuclear changes are shown in the anaplastic astrocytoma in the image below.
There is extreme pleomorphism of these large, irregular, dark, bizarre nuclei. Often, this pleomorphism is so extreme that giant cells are seen ( arrow). However, these cells resemble both the other neoplastic astrocytes in this image and also non-neoplastic reactive astrocytes . The points of resemblance are the homogeneous eosinophilic cytoplasm and the formation of processes--i.e.. extensions of the cytoplasm. The degree of cellularity is also important in determining the high grade of malignancy.
Another indication of malignancy is vascular proliferation. Endothelial proliferation of a vessel in a glioblastoma multiform is seen in the image below- arrow.
The hyperplastic vessels are very often simply very minute lumens embedded in a thick collar of fibroblasts and vascular smooth muscle. The source of the proliferating vessels or their connective tissue matrix has been much debated.
One might think that vascular hyperplasia improves the nutrition of the tumor. But, in fact, the lumens are so small that this contributes, along with the increase in total vascular length, to an increase in vascular resistance and probably to decreased blood flow in the tumor. One might even speculate that this contributes to the necrosis which is characteristic of grade 4 astrocytomas [glioblastomas] illustrated below.
Note the irregular, necrotic, central area surrounded by a palisade of tumor cells. This is called pseudopallisading . Much of the necrosis [ which denotes the highest degree of malignancy (grade IV) ] .is presumed to be due to the fact that the tumor is growing so rapidly that it has outstripped its blood supply. There may also be a role for apoptosis.
We have just discussed and illustrated the two extremes in histological characteristics of astrocytomas: first the grade l tumor with less rapid growth potential; and then the grade III-IV (glioblastoma) with extremely rapid growth. Between these extremes is the grade II astrocytoma, with intermediate cellularity, pleomorphism and anaplasia. Grade II tumors have an intermediate growth potential, but like grade I tumors they can change during their life history into much more rapidly growing grade III-IV tumors.
EPENDYMOMAS
The ependymomas are predominantly tumors of childhood and adolescence and account for 6% of all neuroectodermal tumors. Survival usually does not exceed a few years. They arise most frequently in the fourth ventricle and may cause hydrocephalus by blocking off the normal flow of CSF. However, they can occur anywhere in relation to the ventricular system or central canal and are the most common glioma in the spinal cord and filum terminale. In the spinal cord, they are somewhat well circumscribed and can sometimes be "shelled out" surgically.
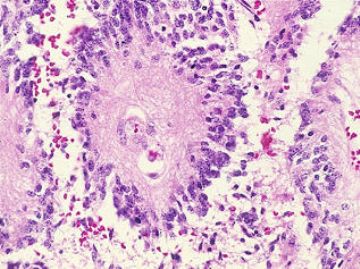
Microscopically, they are composed of elongated cells that have been designated as "ice cream cone," "tadpole," or "carrot" cells because of their tapering shape (image above). The nucleus is located at the large end and mimics the basal location of the nuclei in normal ependymal cells.
These features are seen best in the cells that are arranged in the true rosettes or "miniature ventricles" so clearly seen in the figure. However small "nuclear free zones" as seen in the lower right corner of the figure above are far more frequent than true rosettes and should help the pathologist toward the diagnosis of ependymoma. These "nuclear free zones" are thought to be a consequence of the tendency of the tumor cells to line up around blood vessels to form "pseudorosettes". However [image below] in these cases there is abundant cytoplasm between the tumor cell nuclei and the blood vessel. So much so, that tangential cuts through these structure may show large , pink [eosinophilic] fields without nuclei [the commonly found "nuclear fee zones".
Very anaplastic examples (grade III-IV, ependymoblastomas) are infrequently encountered.
CHOROID PLEXUS PAPILLOMA
A tumor that is related to ependymomas is the choroid plexus papilloma. It is benign although choroid plexus carcinomas do exist.
COLLOID CYST
A tumor formerly thought to be related to ependymoma or choroid plexus is the colloid cyst of the third ventricle (image below). However electronmicroscopy has shown that in some specimens the cyst lining most closely resembles bronchial mucosa which suggests a derivation from displaced bronchial cleft tissue. In any case , this benign tumor can kill if it obstructs the foramina through which CSF passes from lateral to third ventricle. This will suddenly raise intracranial pressure causing severe headache and possible death. Since the tumor is often on a stalk, it may intermittently obstruct the passage of CSF, depending upon the exposition of the patients head. Therefore positional, severe headache is an important clue to this potentially life threatening but curable condition.
OLIGODENDROGLIOMAS
These tumors are usually found in the cerebral hemispheres of middle aged adults and comprise about 5% of neuroectodermal tumors. They grow fairly slowly and there is a mean 5-year survival rate. The tumors may appear more circumscribed than astrocytomas.
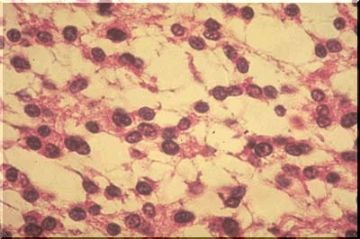
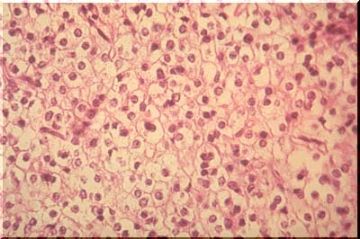
The cells are very uniform and have been described as "fried egg" or "fish eye" cells because of a round, central nucleus surrounded by a clear space or halo (unstained cytoplasm, image above). This appearance depends upon fixation or upon delayed fixation and will not be seen in immediately frozen tissue. Note that the cell membranes are very distinct, giving a honeycomb appearance to the sheets of tumor cells. The tumor cells often occur in nests compartmentalized by delicate blood vessels and their associated connective tissue ["chicken wire"] [Image below]
This tumor also has a tendency to calcify which is sometimes helpful in its radiological diagnosis. Malignant examples (grade III-IV, oligodendroblastomas) are sometimes encountered. As noted earlier some oligodendrogliomas are characterized by deletions in chromosomes 1 and 19. Some oligodendrogliomas contain small cells that have pink cytoplasm and look like small reactive astrocytes of the gemistocytic variety. This has led to including these tumors in the group of mixed astro-oligodendglioma. Such mixed tumors do exist and the prognosis is the more ominous one associated with malignant astrocytomas. However in such mixed tumors the astrocytic component is not restricted merely to small, so-called, "minigemistocytes". When only the latter are present the tumors are now known to behave like oligodendrogliomas and are now simply called oligodendrogliomas.
MEDULLOBLASTOMA
All of the previous tumors that we have discussed have been of glial origin (gliomas). Tumors of neuronal origin are rare and the only important intracranial tumor of this group is the medulloblastoma (neuroblastoma). The term medulloblastoma is a misnomer, since a medulloblast has never been isolated, but the name is too well entrenched to be eradicated.
Most workers think that these tumors arise from identical cells in one of two sites. First, from migrating neuroblasts which have become arrested in the roof of the fourth ventricle (anterior medullary vellum). This would account for the midline location of many of the tumors. Second, from the external granular layer of the cerebellum. The migrating cells form this layer in the immature cerebellum before entering the deep cerebellar cortex (granular layer). The external granular layer disappears (through inward migration) by the second year of life. Because of its origin from immature cells, it is not surprising that the majority of medulloblastomas are seen during the first decade of life. However, there is another peak in young adulthood. These tend to be more laterally placed and have a better prognosis. The fact that some medulloblastomas may simultaneously differentiate along astrocytic lines is another reason that some pathologists retain the name "medulloblastoma."
Since the cells of origin are destined for the cerebellum, medulloblastomas are posterior fossa tumors usually located in the midline of the cerebellum as indicated above. The tumor fills the fourth ventricle (image above, arrows) and characteristically invades the subarachnoid space and seeds up and down the cerebrospinal fluid pathway. This accounts for a generally poor prognosis, though survival is vastly improved following heavy, total neuraxis irradiation. In the image below a huge mass of tumor cells is seen in the subarachnoid space (arrows) and down the spinal cord.
These tumor cells occur in highly cellular sheets which resemble the small, round, lymphocyte-like granular neurons of the normal cerebellar cortex (image below).

- 赚点散碎银子养家,乐呵呵的穿衣吃饭