| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
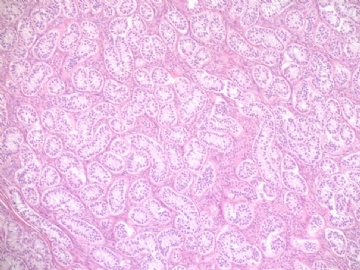
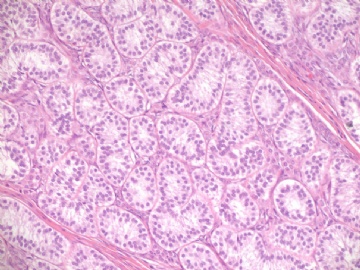
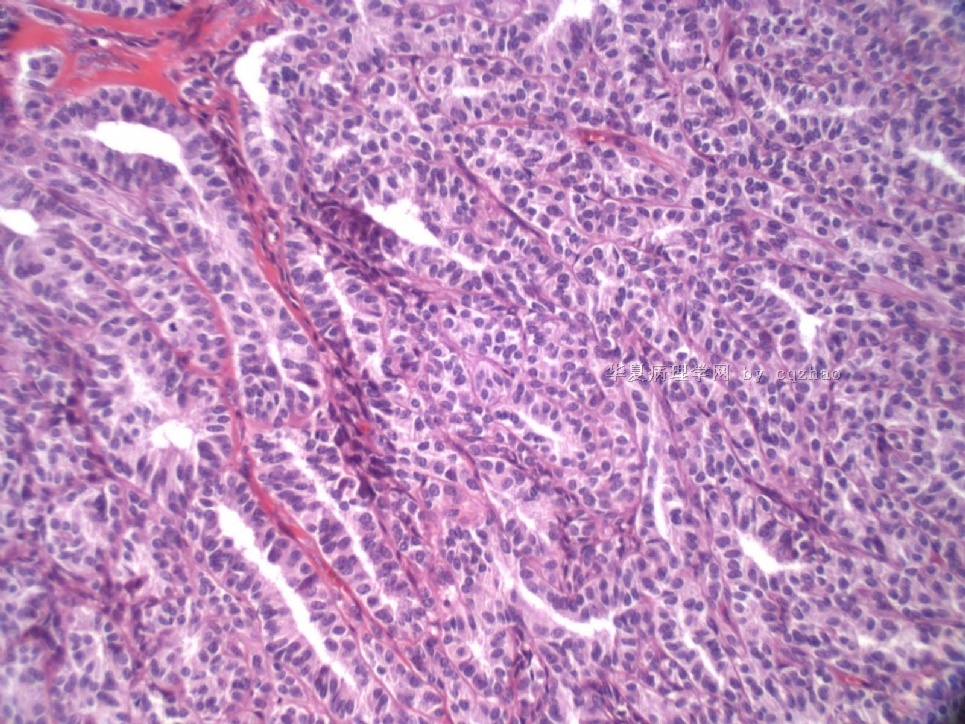
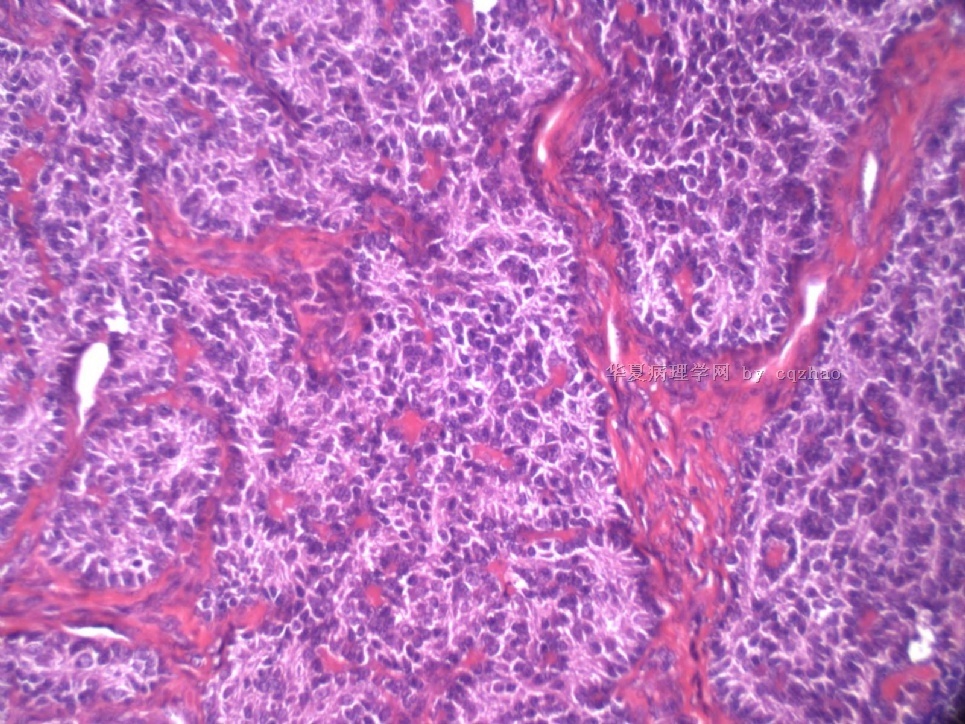
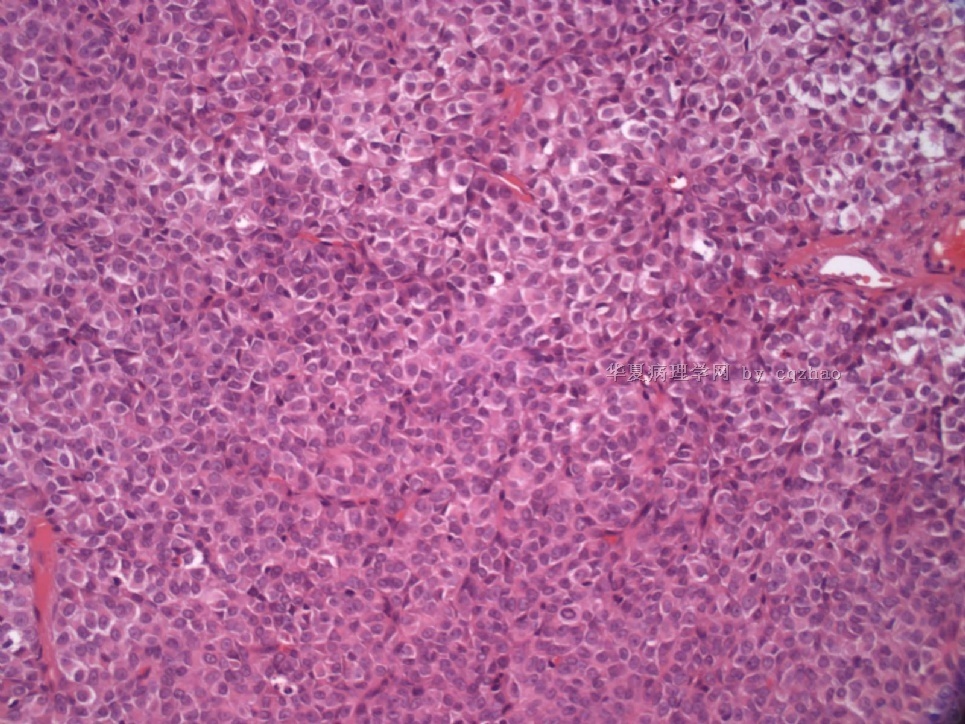
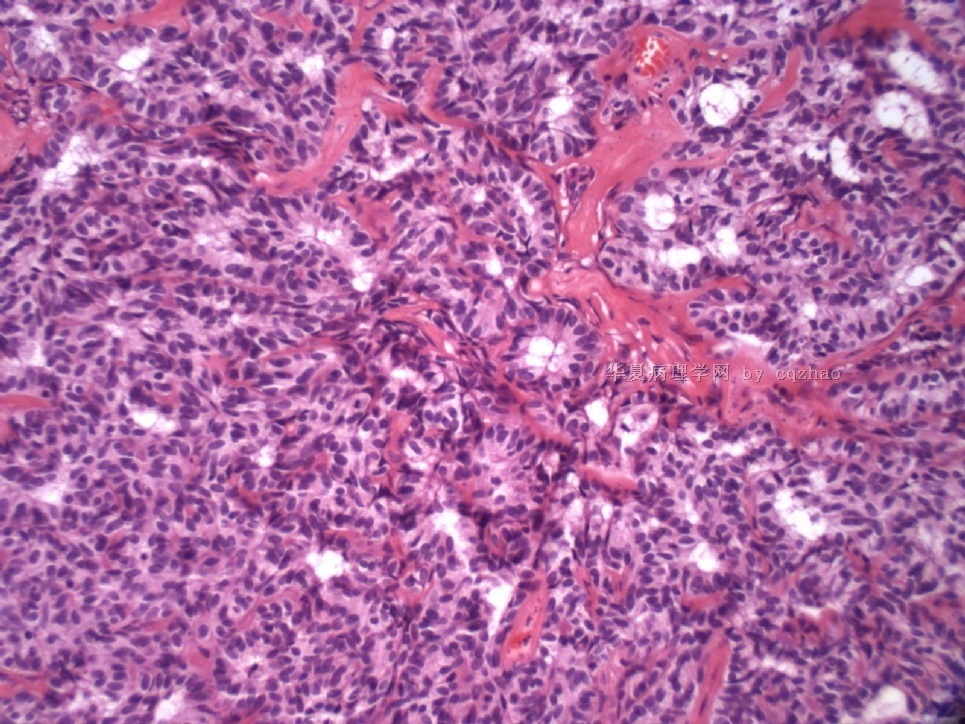
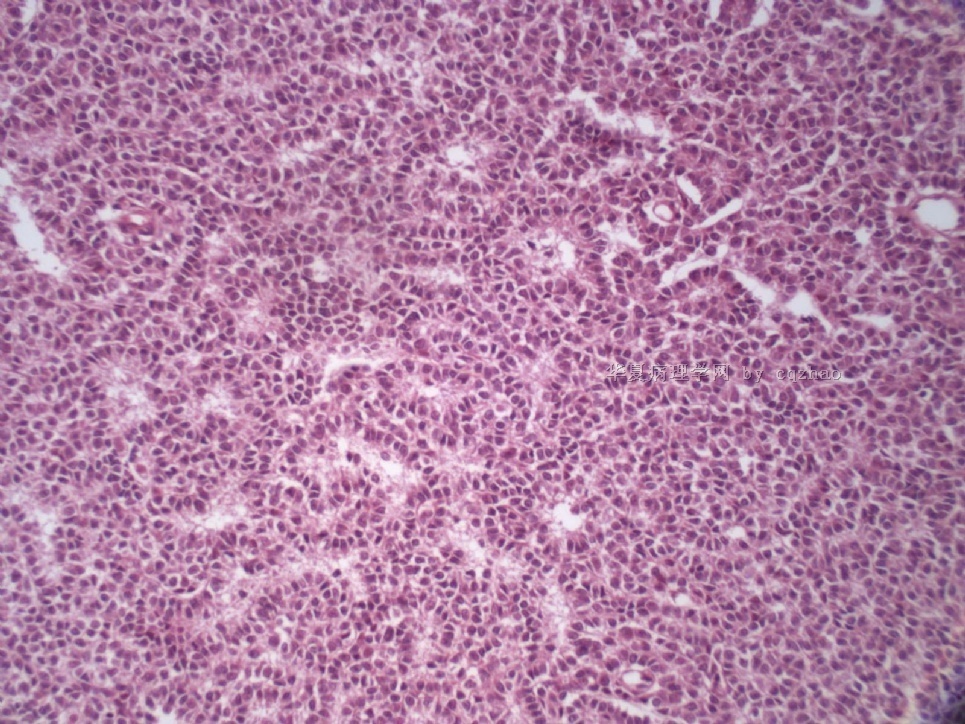
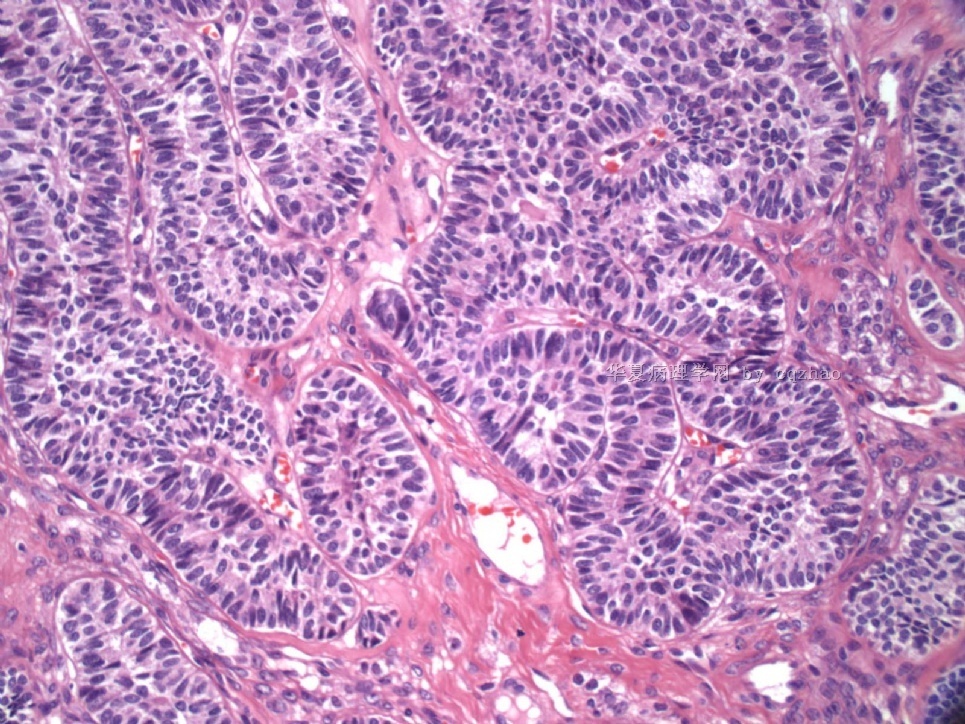
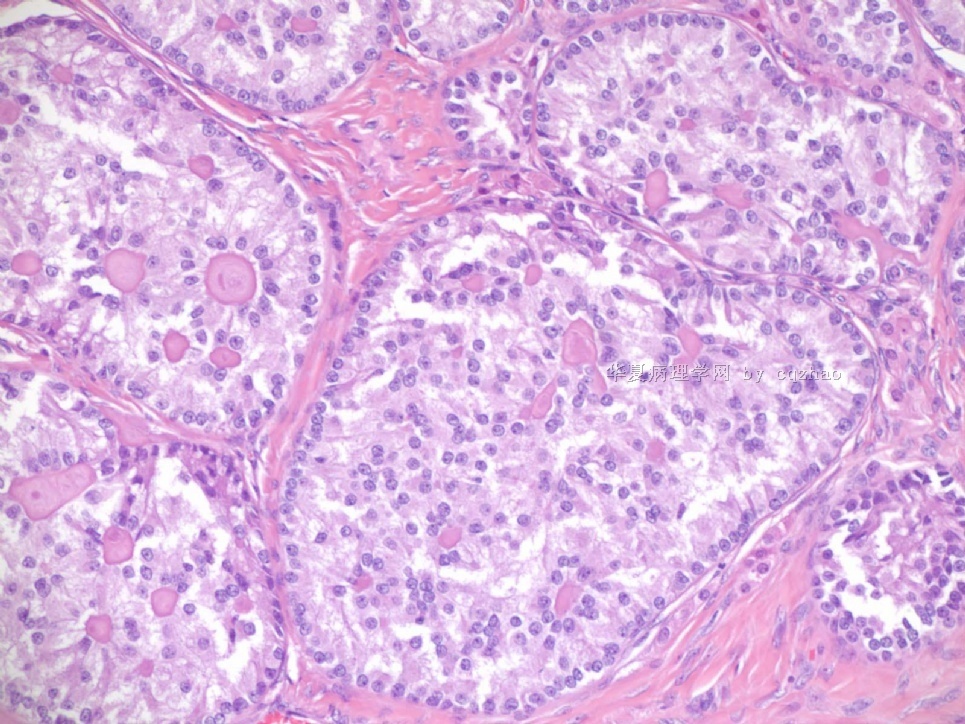
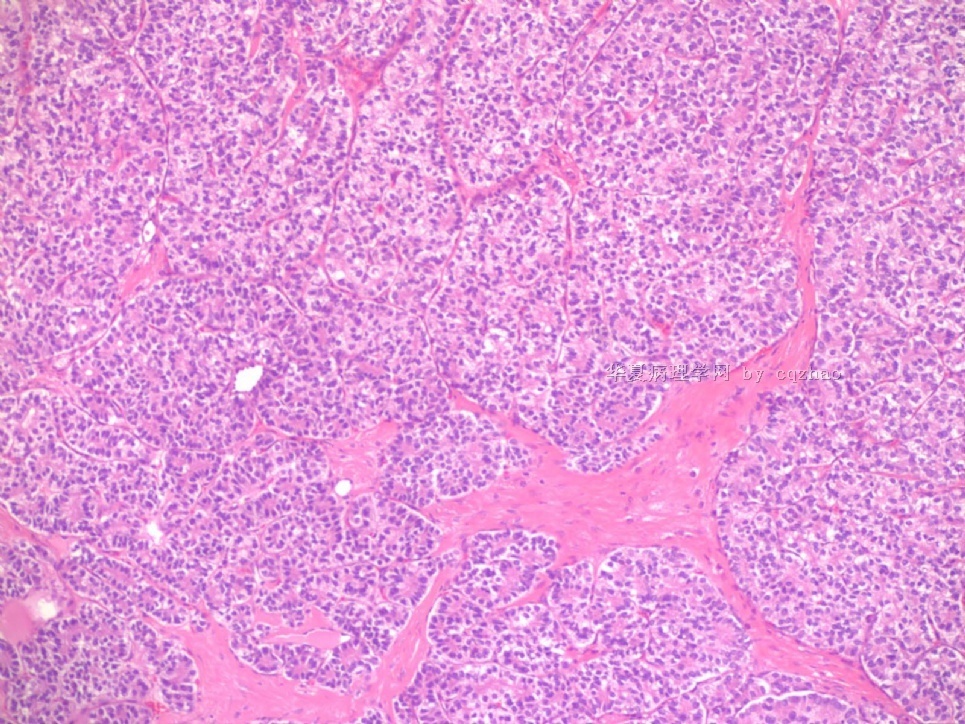
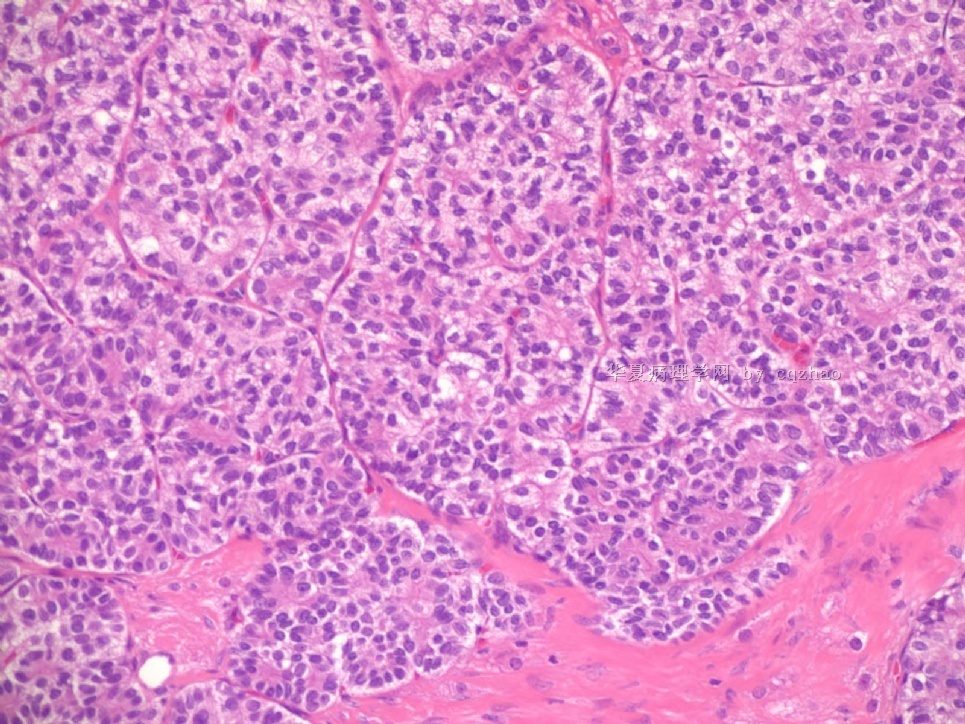
- Sharing some photos of ovarian pure sertoli cell tumors
-
本帖最后由 于 2009-08-03 07:43:00 编辑
You cannot make your dx based on the photos. The purposes that I shared the photos here are I hope people can know the rare tumors like sertoli cell tumors can have variable growth patterns, but not only some clssic patterns. For the true cases you need to know patients' clinical information, tumor gross features, many sections of H@E slides, immunostains, even some molecular methods.
Thanks, cz
-
本帖最后由 于 2009-08-03 07:53:00 编辑
Wish every one who read these photos understand that one category of ovarian sex cord stromal tumor (SCST) can have many different growth patterns, also various ovarian SCST can share similar growth pattern. Of cause this 现象can occur in almost all other tumors also. I may paste the web some cases with IHC in future.
-
本帖最后由 于 2009-08-03 07:45:00 编辑
Ovarian sex cord stromal tumors are rare and may be difficult to make dx. Pure sertoli cell tumors (SCTs) may have variable growth patherns, such as open or solid tubules (most common), cords, trabeculae, solid or diffuse, retiform, pseudopapillary, alveolar, islands, spindled, glandular, lipid, oxyphilic et al. The common differential dx include endometrioid carcinoma, especially sertoli form endometrioid carcinoma, carcinoid tumor (primary or metastatic), metastatic tubular Krukenberg tumor, mesonephric tumor, sepcially female adnexal tumors of probably Wolffian origin (FATWO), Yolk sak carcinoma, and other ovarian epithelial tumors et al.
IHC stains are very important for the dx of ovarian sex cord stromal tumors.
-
本帖最后由 于 2009-07-30 02:13:00 编辑
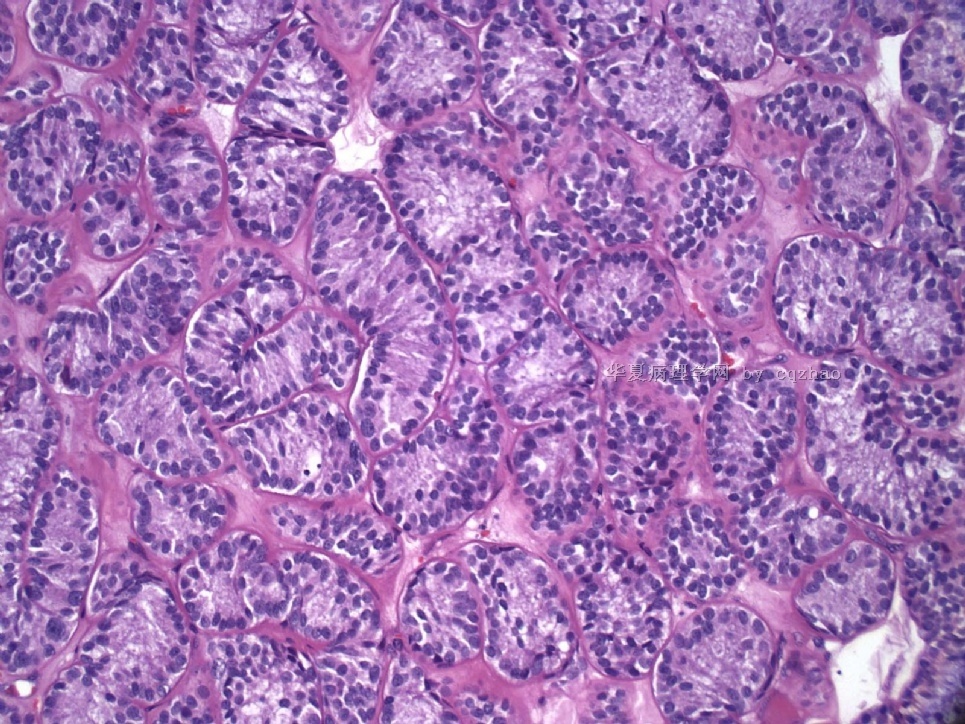
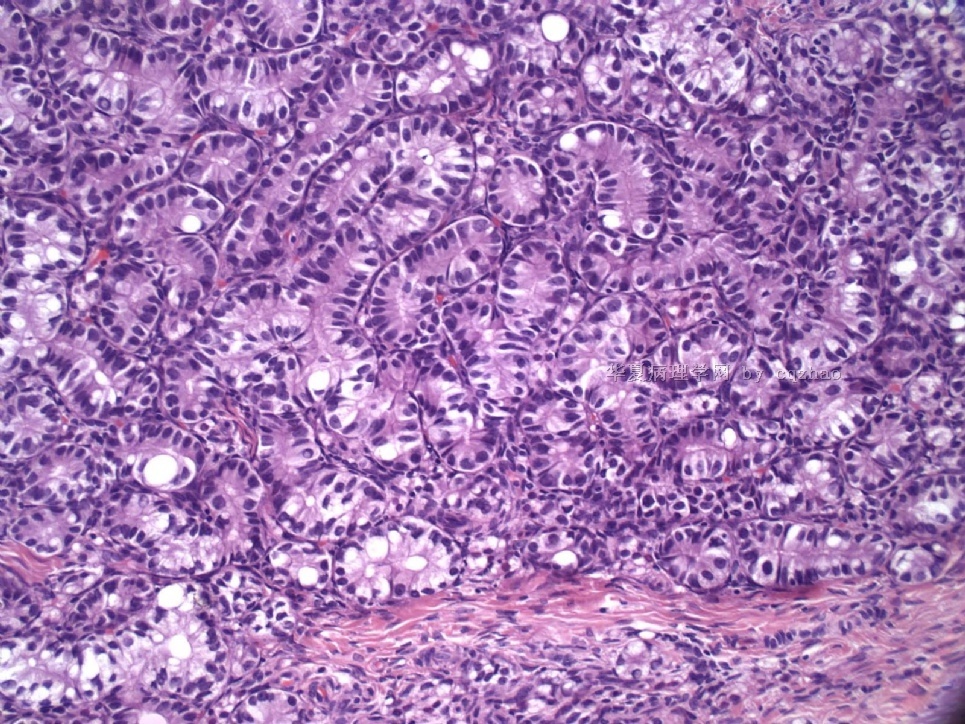
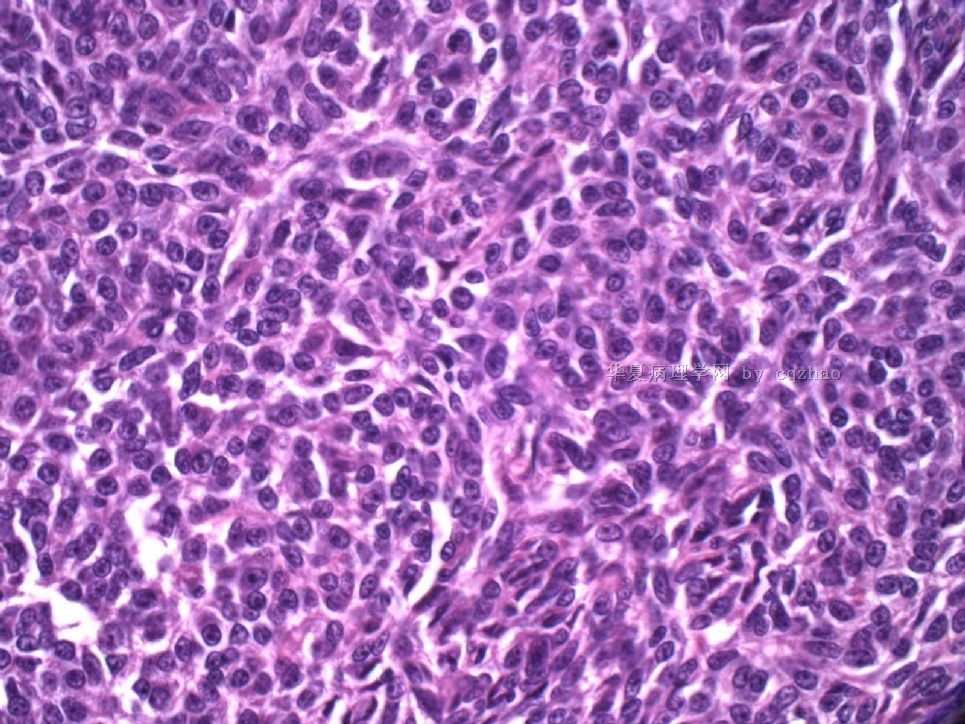
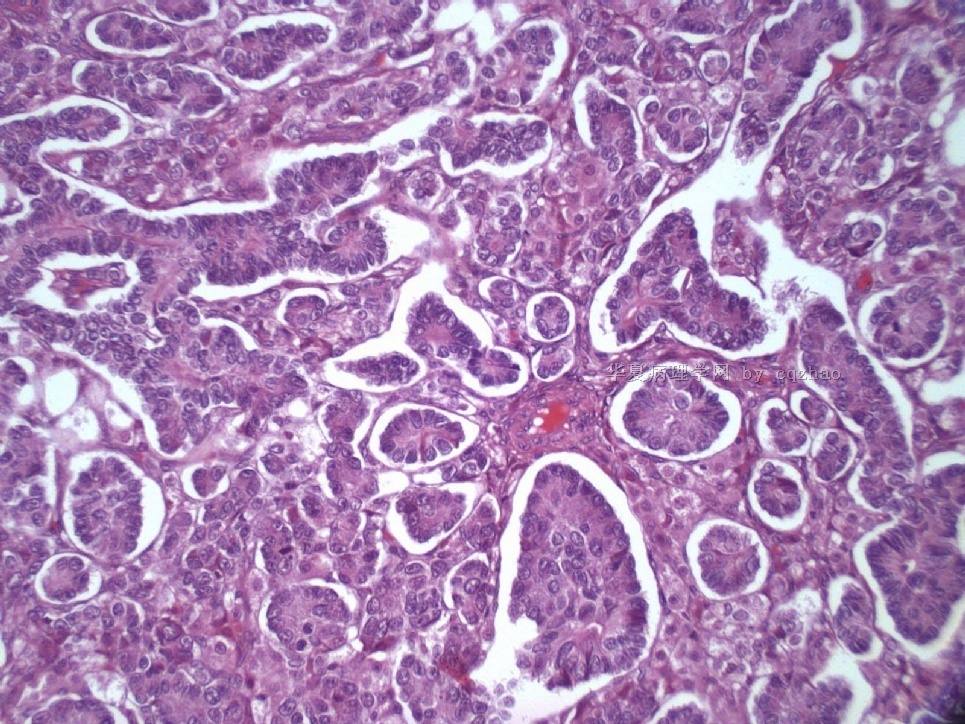
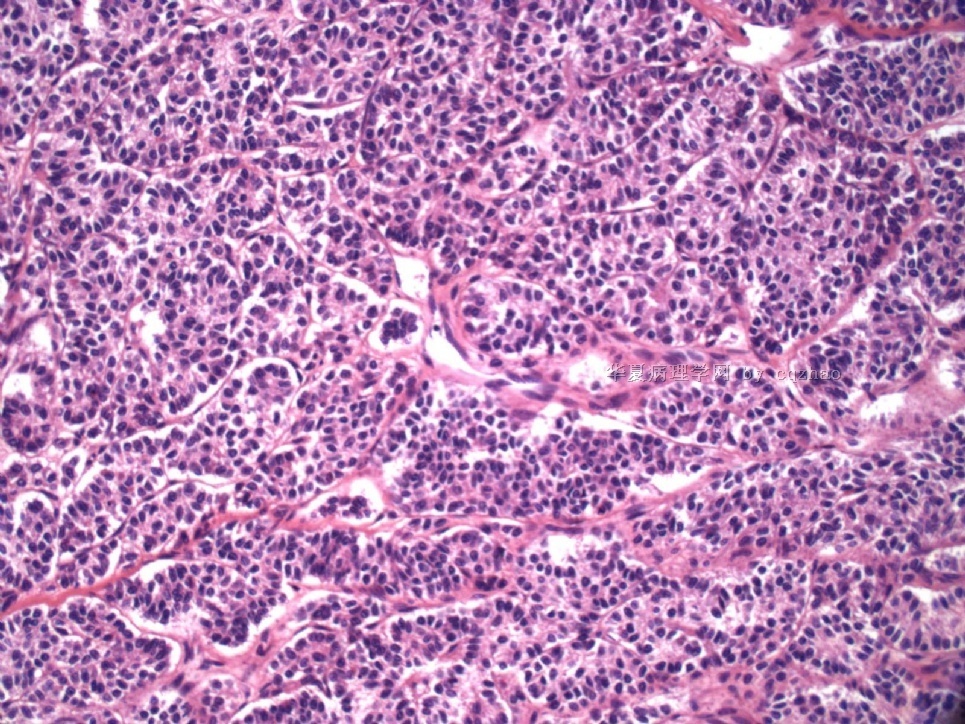
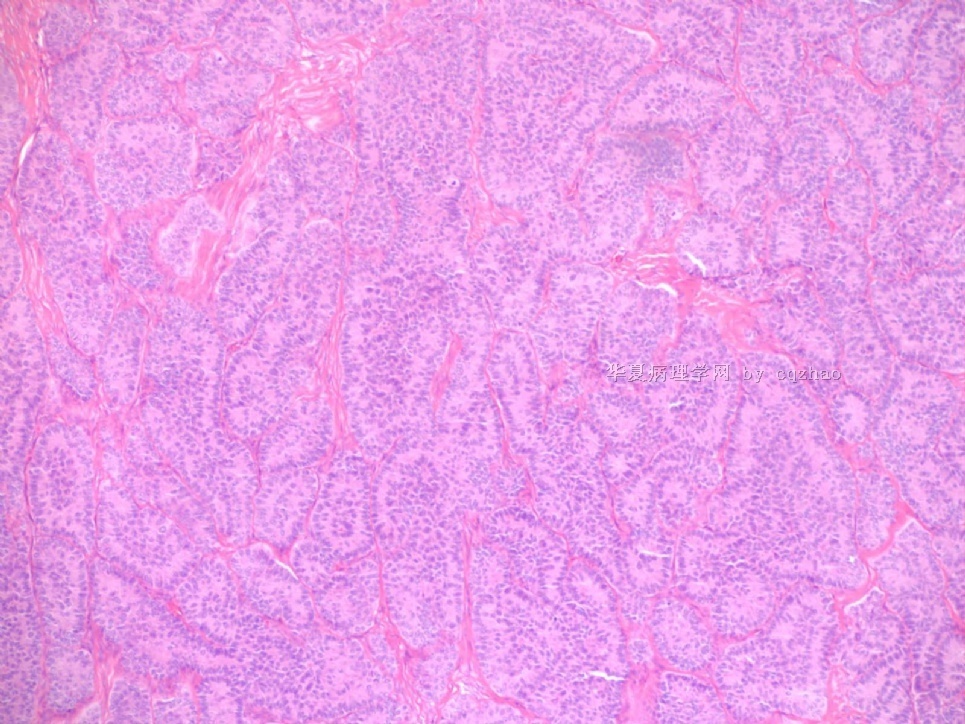
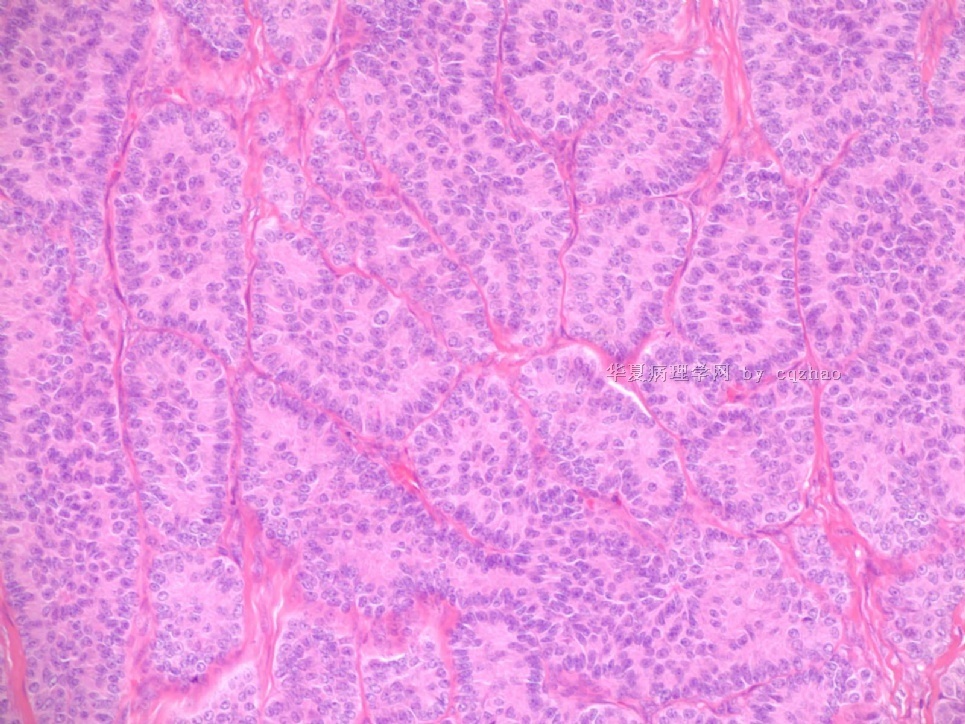
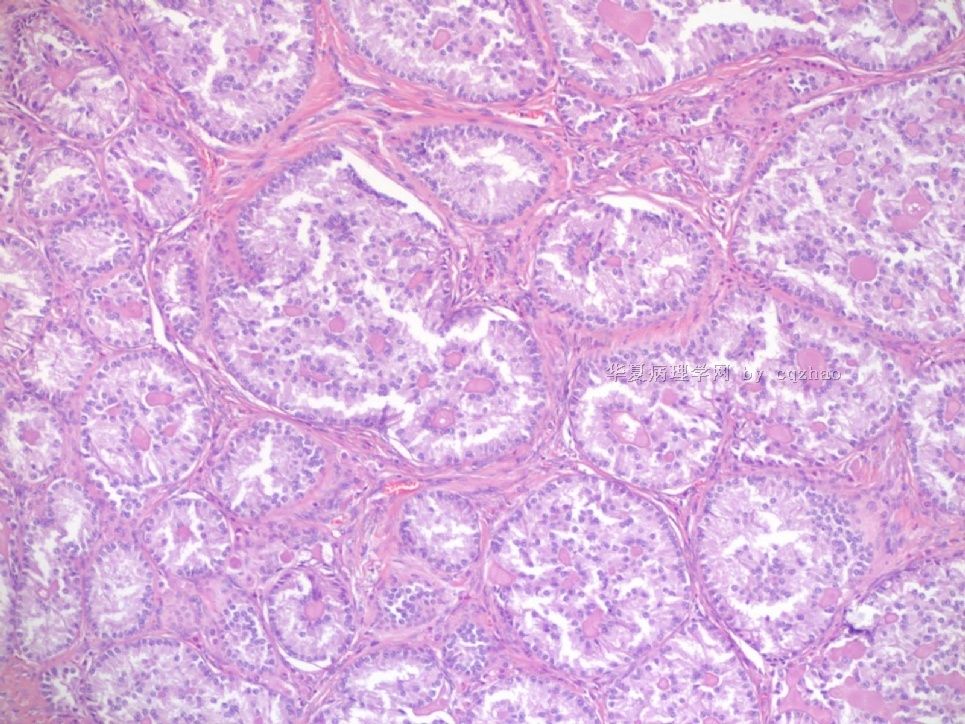
Pure ovarian sertoli cell tumors are rare. I am pasting here another 22 cases of pure sertoli cell tumors. All of these cases are AFIP consult cases since 1970 years. I choose them from my study files. Please enjoy different growth patterns of the tumors and do not question the dx. All of these cases were reviewed by at least three gyncologic pathologists and were stained for more than 20 immuno markers.
I am sure that you are the one of a few pathologists in the world who have seen so many cases of ovarian pure sertoli cell tumors.
Each photo represents one case with 100 or 200 x or 400x
-
Department of Pathology, Magee-Womens Hospital, University of Pittsburgh Medical Center, Pittsburgh, PA 15213, USA. zhaoc@upmc.edu
Different immunohistochemical sex cord-stromal markers have been previously studied in various types of ovarian sex cord-stromal tumors; however, the sensitivity for sex cord-stromal lineage may vary between markers, and some markers may not be as sensitive in some types of sex cord-stromal tumors compared with other tumors in this spectrum of neoplasms. The goals of this study were to determine which immunohistochemical markers are the most sensitive and immunohistochemically robust for sex cord-stromal lineage within a given type of ovarian sex cord-stromal tumor, and to establish whether there are substantial differences of expression of these markers between different types of sex cord-stromal tumors. Immunohistochemical stains for markers which have known variable specificity for sex cord-stromal lineage [inhibin, calretinin, MART-1/melan-A, CD99, steroidogenic factor 1 (SF-1, adrenal 4-binding protein), and WT1], were performed in 127 cases of 5 different types of ovarian sex cord-stromal tumors: adult granulosa cell tumor (n=32), Sertoli cell tumor (n=27), Sertoli-Leydig cell tumor (n=18), steroid cell tumor (n=25), and fibroma/fibrothecoma (n=25). All cases in each type of sex cord-stromal tumor expressed SF-1. Inhibin and calretinin were expressed in all groups of tumors but with a lesser frequency (56% to 100% and 36% to 100% of cases, respectively). All types of tumors except steroid cell tumor expressed WT1. Fibroma/fibrothecoma was the only type of tumor that did not express CD99. The only tumor groups that showed expression of MART-1 were Sertoli-Leydig cell tumor (restricted to the Leydig cell component) and steroid cell tumor (94% and 96% of cases, respectively). The type of sex cord-stromal tumor that was least frequently positive for several of the different markers studied was fibroma/fibrothecoma. Among all tumor groups combined, inhibin and WT1 were the 2 markers showing the most diffuse expression. Likewise, the single marker showing the most optimal combination of diffuse and strong staining (immunohistochemical composite score: possible range, 1 to 12) varied between tumors: adult granulosa cell tumor-inhibin (score 10.0); Sertoli cell tumor-WT1 (score 10.8); Sertoli-Leydig cell tumor (Sertoli cell component)-WT1 (score 10.4); steroid cell tumor-inhibin (score 11.2); and fibroma/fibrothecoma-WT1 (score 8.9). We conclude that most immunohistochemical sex cord-stromal markers have sufficient sensitivity for sex cord-stromal lineage. Although each of the different types of sex cord-stromal tumors has a slightly unique immunoprofile in terms of frequency and extent of expression, these differences are relatively minor for most types of tumors with certain exceptions (eg, WT1 is not diagnostically useful in steroid cell tumor; CD99 is not diagnostically useful in fibroma/fibrothecoma; the only sex cord-stromal tumor for which MART-1 is diagnostically useful is steroid cell tumor; inhibin and calretinin are less diagnostically useful in fibroma/fibrothecoma than in the other types of tumors, but expression in fibrothecoma was higher than in fibroma). SF-1 is the most sensitive sex cord-stromal marker among the most common types of sex cord-stromal tumors. Given the findings relating to sensitivity and extent of expression in this study, and known specificity in the literature, the most informative sex cord-stromal markers to be used for the distinction from nonsex cord-stromal tumors are inhibin, calretinin, SF-1, and WT1 (the exact number of markers to be used should be based on the degree of difficulty of the case and level of experience of the pathologist); however, the utility of immunohistochemistry for the diagnosis of fibroma/fibrothecoma is somewhat limited.
: Am J Surg Pathol. 2009 Mar;33(3):354-66.
Identification of the most sensitive and robust immunohistochemical markers in different categories of ovarian sex cord-stromal tumors.
-
Am J Surg Pathol. 2007 Feb;31(2):255-66.
-
Comparative analysis of alternative and traditional immunohistochemical markers for the distinction of ovarian sertoli cell tumor from endometrioid tumors and carcinoid tumor: A study of 160 cases.
Department of Gynecologic and Breast Pathology, Armed Forces Institute of Pathology, Washington, DC, USA. chengquanzhao@yahoo.com
The main neoplasms in the differential diagnosis for primary ovarian tumors with a tubule-rich pattern are pure Sertoli cell tumor, endometrioid tumors (including borderline tumor, well-differentiated carcinoma, and the sertoliform variant of endometrioid carcinoma), and carcinoid tumor. Because traditional immunohistochemical markers [pan-cytokeratin (pan-CK), low molecular weight cytokeratin (CK8/18), epithelial membrane antigen (EMA), inhibin, calretinin, CD99, chromogranin, and synaptophysin] can occasionally have diagnostic limitations, the goal of this study was to determine whether or not any alternative markers [cytokeratin 7 (CK7), estrogen receptor (ER), progesterone receptor (PR), CD10, and CD56] have better diagnostic utility when compared with traditional markers for this differential diagnosis. Immunohistochemical stains for alternative, as well as traditional, markers were performed on the following primary ovarian tumors: pure Sertoli cell tumor (n = 40), endometrioid borderline tumor (n = 38), sertoliform endometrioid carcinoma (n = 13), well-differentiated endometrioid carcinoma (n = 27), and carcinoid tumor (n = 42). Extent and intensity of immunostaining were semiquantitatively scored. In addition, immunohistochemical composite scores (ICSs) in positive cases were calculated on the basis of the combination of extent and intensity scores. Cytokeratin 7 (CK7) was positive in 97% of endometrioid tumors, 13% of Sertoli cell tumors, and 24% of carcinoid tumors. The differences in the mean ICSs for endometrioid tumors versus Sertoli cell tumor or carcinoid tumor were statistically significant (P values ranging from <0.001 to 0.018). ER and PR were positive in 87% and 86% of endometrioid tumors, 8% and 13% of Sertoli cell tumors, and 2% each of carcinoid tumors, respectively. The differences in the mean ICSs for endometrioid tumors versus Sertoli cell tumor were statistically significant (P values ranging from <0.001 to 0.012). Among the epithelial markers, EMA seemed to be the most discriminatory but only slightly better than CK7, ER, or PR. Pan-CK and CK8/18 were not helpful. CD10 showed overlapping patterns of expression in all categories of tumors. Among the sex cord markers, CD10 was markedly less useful than inhibin or calretinin; CD99 was not discriminatory. CD56 showed overlapping patterns of expression in all categories of tumors. Among the neuroendocrine markers, CD56 was less useful than chromogranin or synaptophysin. When traditional immunohistochemical markers are problematic for the differential diagnosis of ovarian Sertoli cell tumor versus endometrioid tumors versus carcinoid tumor, adding CK7, ER, and/or PR to a panel of markers can be helpful. Endometrioid tumors more frequently express CK7, ER, and PR and show a greater extent of immunostaining in contrast to Sertoli cell tumor and carcinoid tumor. Compared with traditional epithelial markers, CK7, ER, and PR are nearly as advantageous as EMA. Inhibin is the most discriminatory sex cord marker, and CD10 is not helpful in the differential diagnosis. Chromogranin and synaptophysin are excellent discriminatory markers for carcinoid tumor, and CD56 is neither sufficiently sensitive nor specific enough for this differential diagnosis to warrant its use in routine practice.
-
本帖最后由 于 2009-07-28 05:37:00 编辑
-
James Wright Pathology Laboratories, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA. eoliva@partners.org
Am J Surg Pathol. 2005 Feb;29(2):143-56.![]()
Sertoli cell tumors of the ovary: a clinicopathologic and immunohistochemical study of 54 cases.
Ovarian Sertoli cell tumors are rare, and their morphologic spectrum, behavior, and factors influencing the latter are not clearly established. They may be mimicked by many different tumors, some of them more frequent than Sertoli cell tumors; immunohistochemistry may aid in this differential, but its role has not been analyzed in a large series. We studied the clinicopathologic features of 54 Sertoli cell tumors, including the immunohistochemical profile of 23 of them. The patients, 6 of whom had Peutz-Jeghers syndrome, ranged from 2 to 76 years of age (mean, 30 years). Eleven patients had estrogenic and 4 had androgenic manifestations. The tumors ranged from 0.8 to 30 cm, with the majority being in the range of 4 to 12 cm. They were all unilateral, usually solid, and often yellow. The predominant microscopic pattern was tubular, seen, albeit often only focally, in all tumors; other patterns were cords or trabeculae (28), diffuse (21), pseudopapillary (4), retiform (3), islands or alveolar arrangements (3), and spindled (3). The tubules were solid or hollow with the former being somewhat more common. Delicate septa were occasionally seen and were conspicuous in areas of one tumor. The stroma was abundant in 15 tumors with marked sclerosis in 4. The cells usually had pale to occasionally densely eosinophilic cytoplasm, but 6 tumors were composed of cells with prominent foamy cytoplasm, falling in the category of "lipid-rich" Sertoli cell tumor, and one had cells with clear non-foamy cytoplasm. Forty-four tumors were stage I (42 of them were stage Ia and 2 were stage Ic), 1 was stage II, 3 were stage III, and 6 were not adequately staged. Follow-up was available for 27 patients with stage I tumors, and all were alive and well at last follow-up except for 2 patients with stage Ia and 1 with stage Ic disease. Those 3 patients had pelvic-abdominal recurrences 18, 36, and 9 months, respectively, after the initial diagnosis. Two of the three clinically malignant stage I tumors had moderate to severe cytologic atypia and brisk mitotic activity (>5 or more mitoses/10 high power fields [HPFs]), and one of these had tumor cell necrosis. Among the 10 clinically benign stage I tumors with more than 5 years of follow-up, only 3 had >5 mitoses/10 HPFs, but none had more than mild cytologic atypia and none had tumor cell necrosis. Two of the three patients with stage III disease had follow-up information and one was alive at 16 months and the second developed splenic metastases 2 years after the initial diagnosis. Two of the three stage III tumors had at least moderate cytologic atypia and brisk mitotic activity. Immunohistochemical stains showed positivity for AE1/3-Cam5.2 in 15 of 23 tumors; Epithelial membrane antigen (EMA) was negative in all the tumors. Inhibin was positive in 18 of 22 tumors, calretinin in 10 of 20, CD99 in 19 of 22, vimentin in 17 of 18, smooth muscle actin in 4 of 18, neuron specific enolase in 8 of 16, S-100 in 2 of 20, and chromogranin was negative in all 21 cases studied. Although Sertoli cell tumors usually have a distinctive tubular pattern that facilitates the diagnosis, other patterns may occasionally predominate, causing confusion with various other primary and metastatic ovarian tumors. EMA, inhibin, and chromogranin represent the most helpful triad of immunomarkers serving to exclude two common mimics of Sertoli cell tumors (endometrioid carcinoma [inhibin-; EMA+; chromogranin-] and carcinoid tumor [inhibin-; EMA+; chromogranin+]). Although CD99 and calretinin are often expressed in these tumors, they are much less specific and not as helpful in the differential diagnosis. Most Sertoli cell tumors are stage I, unilateral, cytologically bland, and clinically benign, but occasional examples are high stage, and about 11% of stage I tumors have worrisome histologic features that may portend an adverse outcome. The tumors typically occur in young females, sometimes children who typically present with sexual precocity, and occasional patients have Peutz-Jeghers syndrome.
-
本帖最后由 城北 于 2017-02-25 10:18:06 编辑
All of them are pure sertoli cell tumors (SCT) from AFIP files I once used them for IHC study (about 20 stains for each cases). I reviewed 150 cases of pure SCT and used 40 cases for IHC study at AFIP.
As some of you mentioned the case 4 may be called sex cord tumor with annular tubules (SCTAT). Some people may call SCT, complex tubular pattern (Tavassoli). Basically they are the same tumors called different tumors by different authours. People though SCTAT may be related with Peutz-Jeghers syndroma (P-Z-S). In fact Dr. Young paper indicated 11% (6/54)of SCT patients had PJS.
Case 5 is SCT with spindle cell growth pattern. You will appreciate the tubular structure if you see the photos carefully.
Dr. Young paper: Among 54 SCT, The predominant microscopic pattern was tubular, seen, albeit often only focally, in all tumors; other patterns were cords or trabeculae (28), diffuse (21), pseudopapillary (4), retiform (3), islands or alveolar arrangements (3), and spindled (3). The tubules were solid or hollow with the former being somewhat more common.
Remeber that tubular structures are present in all SCT even though they can be very focal.
The largest and best paper about morphogolgic description and clinical followup results of SCT is Dr Young's paper.
The largest and detailed IHC studies on SCT are my two papers. I paste these three papers in the following for some ones who may be interested.
For the last case, please observe the cytomorphology and architectures of the tumor more carefully. Did you notice some compacted tubular structures? Did you see granulosa tumors like this one before?
I can tell you that all these tumors are ov sex cord tumors. Just think a little more and give the reasonable guess. Frankly speaking rare people in the world have seen this growth pattern of the tumor. Now you are one of them.