| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
- 你认为HPV 检测有必要吗?
Please share your oppinion:
请分享你的观点
Recently I noticed this topic in another cytology website in China. Let us have a discussion here.
最近我在另一个中国的细胞学网站中注意到这个主题。让我们一起在这里讨论。
1. Are all cervical carcinoma related to HPV infection?
所有的宫颈癌都是由HPV导致的吗?
2. When the women or who should have high risk HPV (hrHPV) testing?
那些女性需要或者什么时候做高危HPV检测呢?
3. What methods to detect hrHPV do you used in your hospitals?
你们医院用的是那种方法检测高危HPV?
4. What should the women do if she has positive hrHPV result?
如果她的高危HPV的结果是阳性的,她们该怎么办?
5. hrHPV testing should be performed for women with AGC?
细胞学结果为AGC的女性应该进行高危的HPV检测吗?
6. Any question, experience or oppinion you have, please share or discuss.
所有以上问题,就你个人的观点或经验来分享和讨论吧。
We as pathologists should know some basic information about HPV even though HPV testing might not be oerformed in your hospital, or the patients might not be able to pay for the test.
即使HPV检测在你们医院没有开展或病人没有经济能力支付这项检测。我们做为病理学医生应该知道一些关于HPV的基本知识。
-
本帖最后由 于 2009-04-06 09:33:00 编辑
-
Lancet Oncol. 2009 Jul;10(7):672-82. Epub 2009 Jun 17.
-
- Comment in:
- Lancet Oncol. 2009 Jul;10(7):643-4.
HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial.
School of Cancer and Imaging Sciences, University of Manchester, Manchester, UK. henry.c.kitchener@manchester.ac.uk
BACKGROUND: Testing for human papillomavirus (HPV) DNA is reportedly more sensitive than cytology for the detection of high-grade cervical intraepithelial neoplasia (CIN). The effectiveness of HPV testing in primary cervical screening was assessed in the ARTISTIC trial, which was done over two screening rounds approximately 3 years apart (2001-03 and 2004-07) by comparing liquid-based cytology (LBC) combined with HPV testing against LBC alone.
-
METHODS: Women aged 20-64 years who were undergoing routine screening as part of the English National Health Service Cervical Screening Programme in Greater Manchester were randomly assigned (between July, 2001, and September, 2003) in a ratio of 3:1 to either combined LBC and HPV testing in which the results were revealed and acted on, or to combined LBC and HPV testing where the HPV result was concealed from the patient and investigator. The primary outcome was the detection rate of cervical intraepithelial neoplasia grade 3 or worse (CIN3+) in the second screening round, analysed by intention to treat. This trial is registered with the International Standard Randomised Controlled Trial Number ISRCTN25417821.
-
FINDINGS: There were 24 510 eligible women at entry (18 386 in the revealed group, 6124 in the concealed group). In the first round of screening 233 women (1.27%) in the revealed group had CIN3+, compared with 80 (1.31%) women in the concealed group (odds ratio [OR] 0.97, 95% CI 0.75-1.25; p>0.2). There was an unexpectedly large drop in the proportion of women with CIN3+ between the first and second rounds of screening in both groups, at 0.25% (29 of 11 676) in the revealed group and 0.47% (18 of 3866 women) in the concealed group (OR 0.53, 95% CI 0.30-0.96; p=0.042). For both rounds combined, the proportion of women with CIN3+ were 1.51% (revealed) and 1.77% (concealed) (OR 0.85, 95% CI 0.67-1.08; p>0.2).
-
INTERPRETATION: LBC combined with HPV testing resulted in a significantly lower detection rate of CIN3+ in the second round of screening compared with LBC screening alone, but the effect was small. Over the two screening rounds combined, co-testing did not detect a higher rate of CIN3+ or CIN2+ than LBC alone. Potential changes in screening methodology should be assessed over at least two screening rounds.
-
FUNDING: National Institute of Health Research Health Technology Assessment Programme.
There are a lot of large clinical trials for the pap and hpv testing, especially in some developing countries. Some studies supported by some biologic companies may have the bias for the companies' interests.
Very interesing data from ARTITIC TRIAL from UK was published in Lancet oncology on line last month.
See abstract below
I agree with above two doctors' oppinion. There are three approach for screening.
1. Pap test for primary screening, HPV testing for some women with atypical squamous cells. Most hospitals in most countries use this approach. I think this is reasonable.
2. Pap test for primary screening. Combined Pap and HPV testing for women 30 or older
3. HPV testing as primary screening test. If HPV positive, Pap test will be performed as the flow chart I mentioned in floor 115.
WHO approves Cervarix for use in developing countries.
The AP (7/9) reported, "The World Health Organization has approved a second cervical cancer vaccine," Cervarix, "made by GlaxoSmithKline, meaning UN agencies and partners can now officially buy millions of doses of the vaccine for poor countries worldwide." The organization "had previously approved Gardasil, a competing cervical cancer vaccine made by Merck & Co. With two cervical cancer vaccines now ready to be bought by donor agencies, officials estimate that tens of thousands of lives might be saved." Data show that over "80 percent of the estimated 280,000 cervical cancer deaths a year occur in developing countries. In the West, early diagnosis and treatment has slashed the disease's incidence." But, Dan Thomas, a spokesman for the Global Alliance for Vaccines and Immunization (GAVI), pointed out that "the vaccines typically cost about $360 for a three-shot dose -- which is far too expensive for poor countries."
According to Dow Jones Newswires (7/9, Stovall), "Just how the Cervarix deliveries will be paid for has yet to be worked out." In a statement, GlaxoSmithKline said, "We're eager to work with our long-term partner GAVI, as well as other private NGOs or governments of developing countries to identify financing mechanisms for the vaccine." Both Cervarix and Gardasil "are designed to prevent infection by human papillomavirus, or HPV, the virus that causes cervical cancer. Reuters (7/9, Hirschler) also covered the story.
Cervarix may be effective against five types of HPV, study indicates.
The AP (7/8) reports, "GlaxoSmithKline PLC said Tuesday a study shows its human papillomavirus vaccine Cervarix was effective against the types of HPV that are most likely to cause cervical cancer, but also fought other strains of the disease." A study of 18,644 women showed that "women who received all three doses of the vaccine provided 92.9 percent protection, and GlaxoSmithKline said there was also evidence the vaccine protects against HPV types 31, 33, and 45." Results of the study "were published in The Lancet."
According to the Dow Jones Newswires (7/8, Stovall), the "study showed for the first time for any cervical cancer vaccine that Cervarix provided significant cross-protection against pre-cancerous lesions not containing the two most common virus types, the UK-based drugmaker said." The Phase III trial, which involved women "aged between 15 and 25 years, from 14 countries across Europe, Asia-Pacific and Latin and North America," also indicated that "additional efficacy could translate into approximately 11 percent to 16 percent extra protection against cervical cancer over and above the protection afforded by efficacy against the two most common types alone."
Reuters (7/8) also notes that the study's researchers said the only efficient way to stop the virus is to also vaccinate boys and men.
-
本帖最后由 于 2009-06-17 03:51:00 编辑
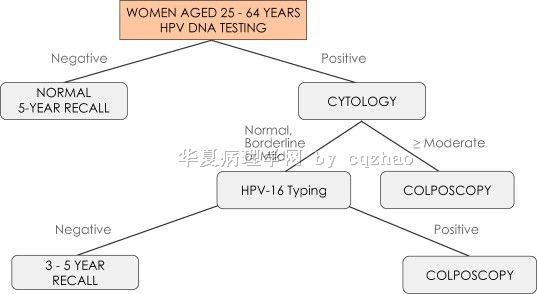
Fig 3: Proposed HPV DNA-based screening algorithm for developing countries
When people talk about cervical cancer screening issue, they always treat China as a developing country.
We have very compicated feeling when we as Chinese American physicians abroad read, know the current cervical ca screening situation in China.
The purpose that I send the proposal for HPV screening here is just to let our young pathologists in China know the new issue in this area. In fact I do not like the proposal , at least now.
-
本帖最后由 于 2009-06-17 03:41:00 编辑
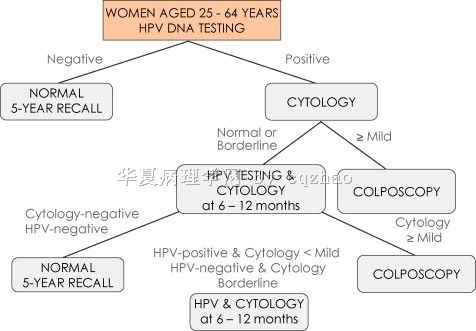
above fig 1: Proposed new screening algorithm which employs HPV DNA testing as the primary screening test and uses cytology to triage HPV positive women.
Fig 2:Proposed potential future screening algorithm. This algorithm employs HPV testing as the primary test and uses cytology to triage HPV positive women to eliminate short follow up group. Triage with p16 and mRNA may also be useful, but this is less well established.
-
本帖最后由 于 2009-06-17 03:32:00 编辑
In fact now some people suggest to use HPV testing as primary screening test. I paste the figs here. The chart figs are from this paper
Vaccine. 2008 Aug 19;26 Suppl 10:K29-41 Comment in: Vaccine. 2008 Dec 9;26(52):6743-4.
Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries.Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, Dillner J, Meijer CJ.
Cancer Research UK Centre for Epidemiology, Mathematics and Statistics, Wolfson Institute of Preventive Medicine, London, United Kingdom. jack.cuzick@cancer.org.uk
名称:图1
描述:图1
名称:图2
描述:图2
名称:图3
描述:图3
-
本帖最后由 于 2009-05-23 21:22:00 编辑
Brief information about HPV
Circular DNA virus
Small virus genome (~8kb)
Capsid proteins – L1 and L2
Oncoproteins – E6 and E7
Deregulated E6/E7
loss of normal cell cycle control
E6 – p53
E7 – RB
oteins – E6 and E7-
本帖最后由 于 2009-05-22 22:20:00 编辑
Good points.
HPV 16/18 account for about 70-80% of cervical cancer.
Current vaccine
HPV L1 VLP vaccine profiles
| Gardasil™ (Merck) | Cervarix™ (GlaxoSmithKline) | |
|---|---|---|
| L1 VLP Antigens | HPV6 20  g g |
HPV16 20  g g |
HPV 11 40  g g |
HPV 18 20  g g | |
HPV 16 40  g g |
||
HPV 18 20  g g |
||
| Expression system | Yeast (Saccharomyces cerevisiae) | Baculovirus |
| Adjuvant | Proprietary aluminum hydroxyphosphate Sulfate (225  g) g) |
ASO4 aluminum hydroxide (500  g) plus 50 g) plus 50  g 3-deacylated monophosphoryl lipid A g 3-deacylated monophosphoryl lipid A |
| Injection volume and immunisation schedule | 0.5 ml i.m. 0, 2 and 6 months | 0.5 ml i.m. 0, 1 and 6 months |
| Adolescent safety/immunogenicity bridging trials | Females and males 9–15 years | Females 10–14 years |
| Males 10–18 years (in progress) | ||
| Licensed | License application made |
i.m.=intramuscular.
-
本帖最后由 于 2009-05-06 09:52:00 编辑
Just notice today's business news for Hologic
May 5 (Reuters) - Shares of Hologic Inc fell 21 percent on Tuesday, a day after the medical diagnostics maker delayed the U.S. launch of its Tomosynthesis mammography system and posted a quarterly loss.
Shares of the company were down to $12.40 in Tuesday pre-market trade from their Monday's close of $15.60 on Nasdaq
-
I spent some time to paste the information in details about the relation of these companies to let more Chinese people know their story. The marketing for Pap test or HPV testing is large, especially in China. 竟争is非常激烈. Just wonder why we do not have ourown products. It is because our government or leaders do not support the issue or some other reasons. Interesting.
1. ThinPrep Pap test is produced by cytyc corporation. The ThinPrep Pap Test is the only liquid-based pap test FDA-approved for HPV. ThinPrep Pap test accounts for 70-80% liquid based Pap cytology marketing in the USA
2. In 2007 Cytyc and Hologic have combined together under the Hologic name.
3. For HPV test: HC2 is only FDA approved test and widely used in the USA. HC2 is the product of DIAGEN.
In 2007 DIAGEN and QIAGEN mergered named QIAGEN.
4. From above news (97 floor) we know now FDA approved Hologic two HPV test products, Cervista(TM) HPV HR (high risk, 14 types) and the Cervista HPV 16/18 tests.
Hologic wants to eat the pie of HPV testing of QIAGEN
- To screen patients with atypical squamous cells of undetermined significance (ASC-US) cervical cytology results to determine the need for referral to colposcopy.
- Used adjunctively with cervical cytology to screen women 30 years and older to assess the presence or absence of high-risk HPV types.
- In women 30 years and older the test may be used adjunctively with the Cervista HPV HR test in combination with cervical cytology to assess the presence or absence of specific high-risk HPV types.
- Used adjunctively with the Cervista HPV HR test in patients with ASC-US cervical cytology results, to assess the presence or absence of specific high-risk HPV types. The results of this test are not intended to prevent women from proceeding to colposcopy.
FDA Approves Two Hologic HPV Tests
BEDFORD, Mass., March 13 /PRNewswire-FirstCall/ -- Hologic, Inc., (Hologic or the Company) (Nasdaq: HOLX - News), a leading women's healthcare company dedicated to serving the healthcare needs of women, today announced that the U.S. Food and Drug Administration (FDA) has approved the Company's premarket approval (PMA) applications for both the Cervista(TM) HPV HR (high risk) and the Cervista HPV 16/18 tests. Cervista HPV HR, designed to detect the 14 high-risk types of human papillomavirus (HPV) known to cause cervical cancer, is the first HPV DNA test approved by the FDA in more than 10 years. Cervista HPV 16/18 is the first HPV test approved for genotyping for HPV types 16 and 18, known to be associated with approximately 70% of all cervical cancers in the United States.
The Cervista HPV HR test has been approved for two uses:
The Cervista HPV 16/18 test has been approved for two uses:
It is also noted that for both the Cervista HPV HR test and the Cervista HPV 16/18 test, the test results, along with the physician's assessment of cytology history, other risk factors, and professional guidelines, may be used to guide patient management.
The Cervista HPV HR clinical trial was one of the largest and most demographically diverse conducted in the United States to date, involving 89 sites and enrolling approximately 4,000 women nationwide. The trial met or exceeded all target endpoints: most importantly, the Cervista HPV HR test achieved 100% sensitivity for the detection of CIN3, an immediate precursor to cervical cancer.
The Company believes Cervista HPV HR provides a number of benefits over current products. Cervista HPV HR includes an internal control intended to verify adequate cellularity for testing, thus reducing the potential for false negative results. In addition, because it requires a smaller specimen volume, this technology may minimize inconclusive, or indeterminate results, which may lead to fewer patients being called back for repeat testing. More importantly, Cervista HPV HR is designed to minimize false positive results due to a low-risk HPV strain being mistakenly recognized as a high-risk HPV strain, thereby potentially reducing unnecessary clinical management and patient anxiety.
The additional approval of Cervista HPV 16/18 marks the first FDA approved HPV genotyping test available in the United States. The HPV strains identified by this test represent the two most oncogenic and persistent types of HPV, together believed to cause approximately 70% of cervical cancer. Previous studies have shown HPV 16 and 18 are respectively 5.5 times and 4.5 times more likely to cause cancer than all other high-risk HPV types combined.
"We are extremely excited to enter this market with such a unique and strong portfolio of FDA approved molecular tests for HPV DNA," said Jack Cumming, chairman and chief executive officer of Hologic. "Our state-of-the-art Cervista HPV tests individually and in combination are designed to provide significant advantages over the existing technology and should help solidify our leadership in cervical cancer screening."
The Cervista HPV HR and Cervista HPV 16/18 tests are based on Invader® chemistry, a patented technology owned by Hologic and well established in other areas of molecular testing. Both tests are approved for use utilizing the sample collected with the ThinPrep® Pap Test, offering additional convenience for the healthcare provider.
About Human Papillomavirus and Cervical Cancer
HPV is the most common sexually transmitted disease (STD) in the United States and is recognized as the cause of most cervical cancers. To help prevent the onset of disease, the American College of Obstetrics and Gynecology (ACOG) suggests routine Pap and HPV testing for women over the age of 30 to identify women most likely to develop cervical cancer.
About Hologic, Inc.
Hologic, Inc. is a leading developer, manufacturer and supplier of premium diagnostics products, medical imaging systems and surgical products dedicated to serving the healthcare needs of women. Hologic's core business units are focused on breast health, diagnostics, GYN surgical, and skeletal health. Hologic provides a comprehensive suite of technologies with products for mammography and breast biopsy, radiation treatment for early-stage breast cancer, cervical cancer screening, treatment for menorrhagia, osteoporosis assessment, preterm birth risk assessment, mini C-arm for extremity imaging and molecular diagnostic products including reagents for a variety of DNA and RNA analysis applications. For more information, visit www.hologic.com.
Hologic, Cervista, ThinPrep and Invader are trademarks and/or registered trademarks of Hologic, Inc. and/or its subsidiaries in the United States and/or other countries.
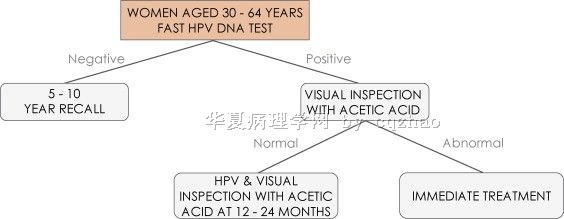
Just feel interested in the new careHPV trial in China and did some literature search and pasted above.
Hope our news or scentific research papers can report study results and significance more objectively. Otherwise they can mislead the readers and geneal people.













