| 图片: | |
|---|---|
| 名称: | |
| 描述: | |
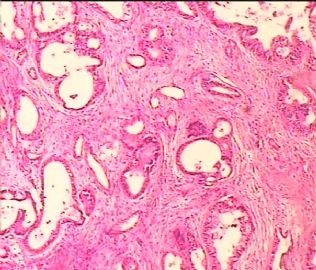
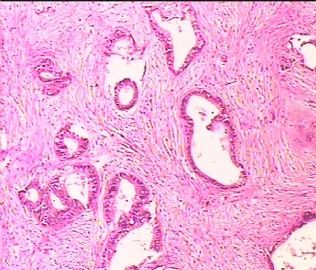
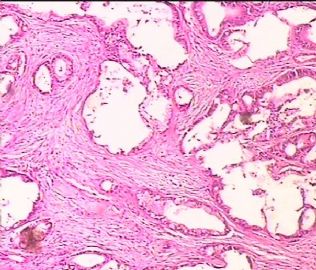
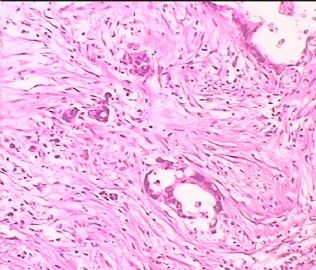
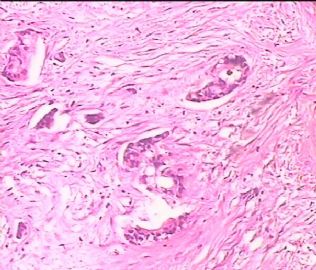
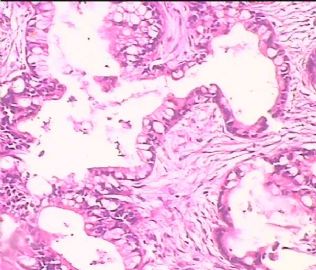
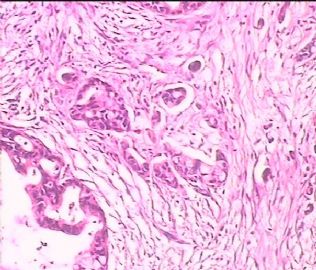
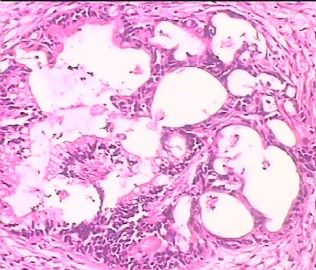
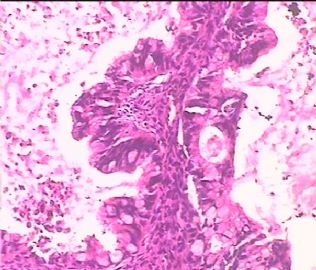
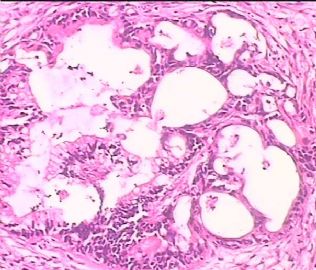
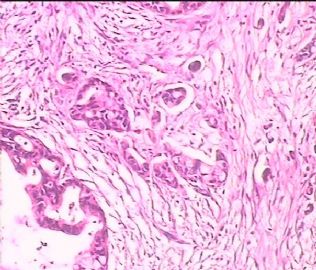
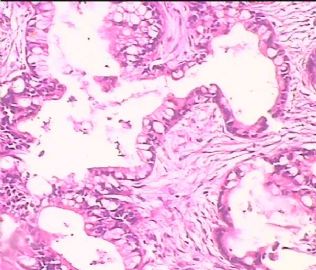
- 双侧卵巢肿瘤 ,请大家来帮帮忙
The key for this case is that you must rule out or rule in the metastatic carcinoma. irregular shaped glands with various size make the metastatic carcinoma very possible.
Different dx:
Primary mucinous tumor
metastatic tumor: lower GI, Upper GI.
add more IHC:
CDX2: positive for low GI and some ovarian mucinous tumor. Often it is weak stain in ovarian mucinous tumor
CK7/ck20: ovary: ck7+/ck20-/CDx2 variable; lower GI: ck7-/ck20/cdx2+; upper GI: usually cytokeratin 7-positive and CDX2/cytokeratin 20 variable
CK19, CA19.9 (pancrease)
CA125
There are some overlapping about the IHC. clinical correlation with Immaging is needed.
There are some new antibodies in the research papers. You may not have them now,
-
Mod Pathol. 2006 Nov;19(11):1421-8. Epub 2006 Sep 15.
-
Immunohistochemical expression of CDX2 in primary ovarian mucinous tumors and metastatic mucinous carcinomas involving the ovary: comparison with CK20 and correlation with coordinate expression of CK7.
Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore, MD 21231, USA. rvang1@jhmi.edu
Recent studies have demonstrated conflicting results regarding the value of CDX2 for distinguishing primary ovarian mucinous tumors from metastatic mucinous carcinomas in the ovary. Utility of coordinate expression of cytokeratins 7 and 20 is restricted to distinction of ovarian mucinous tumors from lower gastrointestinal tract metastases and data comparing coordinate expression of all three markers is limited. Immunohistochemical studies were performed to compare expression of CDX2 and cytokeratin 20, both markers of intestinal differentiation, in conjunction with coordinate expression of cytokeratin 7, in 90 mucinous tumors involving the ovary: 42 primary ovarian mucinous tumors (31 atypical proliferative (borderline) mucinous tumors (gastrointestinal type), 11 mucinous carcinomas) and 48 metastatic mucinous carcinomas of upper (pancreaticobiliary tract: 14; stomach: five) and lower (colon and rectum: 25; appendix: four) gastrointestinal tract origin. Primary ovarian tumors expressed CDX2 (40%) less frequently than cytokeratin 20 (83%) (P<0.0001). CDX2 expression in primary ovarian tumors (40%) was lower than CDX2 expression in metastatic carcinomas of both upper (74%; P=0.016) and lower gastrointestinal tract origin (90%; P<0.0001). Cytokeratin 20 expression was similar in primary ovarian tumors (83%) and metastases of upper (89%; P=0.071) and lower gastrointestinal tract origin (93%; P=0.29). Thus, as a single marker CDX2 offers some advantage over cytokeratin 20 because it is less frequently positive in primary ovarian tumors. In the almost universally cytokeratin 7-positive primary ovarian tumors and metastases of upper gastrointestinal tract origin, CDX2 coordinate expression was less common in primary ovarian tumors (36%) than in metastases of upper gastrointestinal tract origin (63%) (P=0.022) whereas cytokeratin 20 coordinate expression was identical in both tumor types (79%). In the almost universally cytokeratin 7-negative metastases of lower gastrointestinal tract origin, coordinate expression of CDX2 (83%) and cytokeratin 20 (86%) were equivalent (P=1.00). CDX2 was comparable to cytokeratin 20 in distinguishing metastases of lower gastrointestinal tract origin (usually cytokeratin 7-negative and CDX2/cytokeratin 20 positive) from primary ovarian tumors and metastases of upper gastrointestinal tract origin (usually cytokeratin 7-positive and CDX2/cytokeratin 20 variable). CDX2 provided some advantage over cytokeratin 20 for distinguishing primary ovarian mucinous tumors from metastases of upper but not lower gastrointestinal tract origin; however, the advantage in the former was limited due to the occurrence of shared coordinate expression profiles in both tumor types. Cytokeratin 7 provides the predominant discriminatory value among these markers yet is limited to distinction of primary ovarian tumors from metastases of lower gastrointestinal tract origin.
-
Am J Surg Pathol. 2006 Sep;30(9):1130-9.
-
Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases.
Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore, MD, USA. rvang1@jhmi.edu
Coordinate expression profiles for cytokeratins 7 and 20 (CK7 and CK20) are useful for distinguishing certain types of adenocarcinomas but use for distinction of primary and secondary mucinous tumors in the ovary is limited due to the existence of a number of tumor types exhibiting overlapping CK7/CK20 immunoprofiles; the use of staining distribution patterns in the distinction of tumors with shared profiles has not been evaluated in detail. We report analysis of both coordinate expression profiles and staining distribution in 179 rigorously classified mucinous tumors in the ovary, including 53 primary tumors [35 atypical proliferative (borderline) mucinous tumors of gastrointestinal type and 18 invasive mucinous carcinomas] and 126 secondary tumors [28 colorectal adenocarcinomas, 54 appendiceal tumors (23 adenocarcinomas, 31 low-grade adenomatous mucinous tumors associated with pseudomyxoma peritonei), 14 pancreatic adenocarcinomas, 8 endocervical adenocarcinomas, 5 gastric adenocarcinomas, 4 gallbladder/biliary tract adenocarcinomas, and 13 adenocarcinomas of unknown primary sites). A CK7+/CK20+ immunoprofile was the most common profile in primary ovarian tumors (74%), upper gastrointestinal tract tumors (78%), and endocervical tumors (88%) but was occasionally observed in lower intestinal tract tumors (colorectal: 11%; appendiceal: 13% of low-grade tumors, 35% of carcinomas). A CK7-/CK20+ immunoprofile was the most common profile in lower intestinal tract tumors (79%) and was uncommon in upper gastrointestinal tract tumors (9%), rarely seen in primary ovarian tumors (4%), and not seen in endocervical tumors. A CK7+/CK20- profile was observed in some primary ovarian (23%), upper gastrointestinal tract (13%), and endocervical tumors (13%) but not in lower intestinal tract tumors. For CK7+ tumors, staining distribution was very frequently diffuse (>50% of tumors cells positive) in primary ovarian, upper gastrointestinal tract, and endocervical tumors, whereas staining distribution was often focal (<50% of tumors cells positive) when present in colorectal and appendiceal carcinomas but not in low-grade appendiceal tumors. For CK20+ tumors, staining distribution was variable but often focal in primary ovarian tumors and nonlower intestinal tract tumors, whereas the pattern was almost always diffuse in lower intestinal tract tumors. Immunohistochemical staining distribution can supplement CK7/CK20 coordinate expression profiles to distinguish subsets of primary ovarian and metastatic lower intestinal tract mucinous tumors having overlapping immunoprofiles but neither coordinate expression profiles nor staining distribution distinguishes primary ovarian tumors from the nonlower intestinal tract metastases.
-
Cytojournal. 2007 Jun 22;4:13.
-
Immunohistochemical expression of SMAD4, CK19, and CA19-9 in fine needle aspiration samples of pancreatic adenocarcinoma: Utility and potential role.
Department of Pathology and Laboratory Medicine, Emory University Hospital, Atlanta, GA, USA. mzapata@emory.edu
BACKGROUND: Pancreatic adenocarcinoma comprises 85% of all cases of pancreatic malignancies. From a diagnostic standpoint, these tumors are readily diagnosed by fine needle aspiration, with an accuracy of greater than 90%; however it is often difficult to ascertain whether these are primary or metastatic in nature. This study was undertaken to see the usefulness of CK19, CA19-9 and a newly described marker, SMAD4 in confirming the pancreatic origin of these tumors. Briefly, SMAD4 (DPC4) is a tumor-suppressor gene located on chromosome 18q which has been shown to mediate the downstream effects of TGF-beta superfamily signaling, resulting in growth inhibition. The loss of SMAD4, which as been reported to occur in 55% of pancreatic ductal adenocarcinomas may lead to up regulation of cell cycle proteins and hence increase cellular proliferation. In addition, SMAD4 has been suggested to possibly have prognostic potential, with the presence of SMAD4, indicating shorter survival after resection. DESIGN: Clinical data was reviewed to identify patients with proven, primary pancreatic adenocarcinoma. A total of 25 patients with diagnostic material from fine needle aspiration cell blocks, were retrieved from our files at Emory University Hospital. In addition cell blocks from clinically diagnosed non-pancreatic adenocarcinomas were also selected as controls for this study (10 cases of colonic adenocarcinoma, 10 cases of pulmonary adenocarcinoma, 10 cases of breast ductal carcinoma and 10 cases of ovarian mucinous adenocarcinoma). Formalin fixed, paraffin-embedded sections from these were stained with SMAD4, CK19, and CA19-9, using pressure cooker antigen retrieval, labeled polymer HRP (DAKO), and the DAKO autostainer. RESULTS: Immunohistochemical staining was reviewed based on intensity (negative, low-positive, and high-positive) and percentage of cells. In primary pancreatic ductal adenocarcinoma, CK 19 showed diffuse cytoplasmic positivity in 23 of 25 cases, CA 19-9 showed apical cytoplasmic staining in all 25 cases, and SMAD4 showed nuclear staining in 20 of 25 cases. In the control group comprising of non-pancreatic adenocarcinoma SMAD4 was negative (100%) in all 10 cases of colonic and pulmonary adenocarcinoma. However 1 of 10 cases (10%) of breast and ovarian adenocarcinoma did show low positivity nuclear staining. However the expression of CA19-9 and CK19 was more variable in these different non-pancreatic malignancies. CONCLUSION: Pancreatic adenocarcinoma showed positive immunohistochemical staining for SMAD4 in 80%, CK19 in 100% and CA19-9 in 100% of the selected cases. These markers, when used as a panel, may confirm the diagnosis of pancreatic adenocarcinoma in fine needle aspiration samples, and help in differentiating from metastatic adenocarcinoma. This may help in determination of appropriate surgical and chemotherapeutic options.
-
Pancreas. 2008 Jan;36(1):e40-6.
-
Immunohistochemical similarities between pancreatic mucinous cystic tumor and ovarian mucinous cystic tumor.
Department of Surgery, Kyorin University School of Medicine, Tokyo, Japan. ysuzuki@kyorin-u.ac.jp
OBJECTIVE: Pancreatic mucinous cystic tumor (MCT(P)) and ovarian mucinous cystic tumor (MCT(O)) show common features. However, there are few studies showing a comparison of both types of tumor. We immunohistochemically studied both types of tumor to clarify their characteristics. METHODS: Eight patients with MCT(P) and 21 patients with MCT(O) were examined. The tumors were immunohistochemically examined using antibodies against female sex hormone receptors (estrogen receptor, progesterone receptor, and alpha-inhibin), pancreatobiliary tissue markers (carbohydrate antigen 19-9 and DUPAN2) and cell cycle regulators (p27kip1 and phosphorylated retinoblastoma). Samples from 7 female patients with invasive pancreatic ductal carcinoma (DC), 8 female patients with normal pancreatic tissue, and 10 patients with normal ovarian tissue were also examined. RESULTS: In the tumor epithelial cells, the expressions of DUPAN2 and p27/kip1 were similar between MCT(P) (38% and 88%, respectively) and MCT(O) (14% and 76%, respectively), but significantly different between both tumors and DC (100% and 0%). In the stromal cells, the expressions of estrogen receptor, progesterone receptor, alpha-inhibin, and p27/kip1 were similar between MCT(P) (63%, 75%, 50%, and 63%, respectively) and MCT(O) (57%, 71%, 81%, and 57%, respectively), but significantly different between both tumors and DC (0%, 0%, 0%, and 0%, respectively). CONCLUSIONS: MCT(P) and MCT(O) have several immunohistochemical similarities and are significantly different from DC. The female sex hormone system may play a major role in the development of both MCT(P) and MCT(O). A frequent p27/kip1 expression level was associated with nonaggressive progression of both tumors.
-
Histol Histopathol. 2009 Jan;24(1):77-82.
-
Distinctive immunohistochemical profile of mucinous cystic neoplasms of pancreas, ovary and lung.
Department of Pathology, Allegheny General Hospital, Pittsburgh, USA.
Mucinous cystic neoplasms (MCNs) of the pancreas, ovary and lung have a similar histologic appearance. We investigated if immunohistochemical (IHC) studies could help in separating these neoplasms. Twenty-six ovarian MCNs (invasive carcinoma and borderline tumor), 12 pancreatic MCNs (invasive carcinoma, and with moderate or high-grade dysplasia), and 3 pulmonary MCNs (only invasive carcinoma) were retrieved. Our study demonstrated that pancreatic MCNs are positive for CDX-2 (67%), PDX-1 (100%), CK7 (83%) and CK20 (100%), while are negative for CA-125. The IHC profile of ovarian intestinal type MCN is similar to that of pancreatic MCNs, except for lower frequency of CDX-2 expression (29% vs. 67%). Ovarian endocervical like MCNs are positive for CA-125 (100%) and CK7 (100%), while are negative for CDX-2, PDX-1 and CK20. Pulmonary MCNs are positive for CDX-2 (100%), CK7 (100%) and CK20 (100%), while are negative for PDX-1 and CA-125. All tumors are negative for TTF-1, D2-40 and WT-1. We concluded that an IHC panel of CDX-2, PDX-1, CA-125, and CK20 is useful in separating MCNs of the pancreas, ovary and lung.